The question of how to adequately capture the holistic value of orphan drugs (ODs) for rare diseases is the subject of much debate among industry stakeholders including manufacturers, clinical experts, health economists and payers.
This is especially the case in markets using cost-effectiveness analysis (CEA) for their health technology assessments (HTA). The problem is particularly relevant for manufacturers of ODs that may not be able to fulfil conventional assessment criteria but offer the intrinsic value of providing new treatments for rare and often severe diseases.
The CRA Life Sciences Practice team set out to investigate how the value of disease rarity is incorporated into decision-making for ODs in England. We reviewed 47 non-oncology ODs that were approved by the European Medicines Agency (EMA) between 2006 and 2019.
Reimbursement decisions and prices were collected or calculated from reports published by The National Institute for Health and Care Excellence (NICE) or National Health Service (NHS) in England. We focused specifically on exploring the price and access outcomes of orphan medications for chronic conditions.
This type of drug often attracts greater attention from decision-makers due to the potential for significant and long-term budget impact.
The study focused on answering three key questions:
1. Is disease rarity a predictor of reimbursement?
2. Is there a relationship between disease rarity and the cost of treatment?
3. Are drugs for rare diseases disproportionately subject to commercial agreements?

Is disease rarity a predictor of reimbursement?
There are two routes for achieving routine reimbursement for new medicines in England: first, through one of NICE’s technology appraisal frameworks (here, we focus on the single technology appraisal [STA] and the highly specialised technology [HST] frameworks), or, second, via NHS England specialised commissioning.
Manufacturers have historically had the option to apply for assessment by either NICE or NHS England, though the HST framework and specialised commissioning are restricted to ODs.
NICE uses CEA as the primary method for jointly assessing the clinical and economic impact of new drugs. Binary reimbursement decisions are based predominantly on a drug’s adherence to the cost- effectiveness thresholds of each framework. On the other hand, NHS England typically considers four key factors in reimbursement decision- making: disease prevalence, treatment and service cost, number of possible service providers and the financial implications for the clinical commissioning groups (CCGs).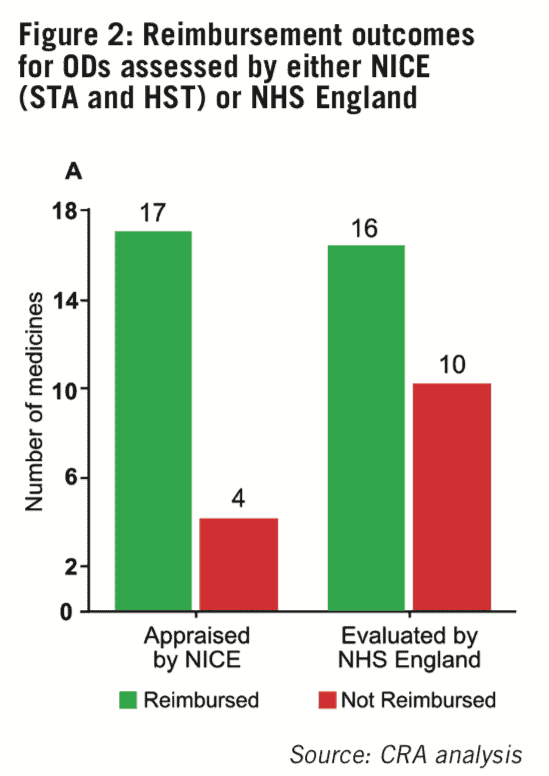
The overall level of reimbursement for non- oncology ODs in England is high, with 70.2% (33/47) of medicines in the sample being routinely reimbursed. However, the likelihood of achieving reimbursement seems to vary between the two routes of assessment, as results showed drugs appraised by NICE were more likely to be recommended for routine reimbursement (80.9%; 17/21) compared with drugs evaluated by NHS England for specialised commissioning (61.5%; 16/26) (see Figure 2).
We used binary logistic regression to determine if disease rarity can be used to predict the likelihood of reimbursement – findings indicate it is not, whether ODs are assessed by NICE or NHS England. Disease rarity is thus not a major driver of reimbursement decisions in value assessments for ODs.
Is there a relationship between disease rarity and the cost of treatment?
We also explored the relationship between disease prevalence (expressed as cases per 10,000 persons) and the average annual treatment cost (AATC – based on list prices) for ODs reimbursed via NICE or NHS England. Findings showed a strong but non-linear relationship between disease prevalence and AATC for medicines recommended by NICE, with ODs for diseases with lower prevalence achieving higher AATCs (see Figure 3A).
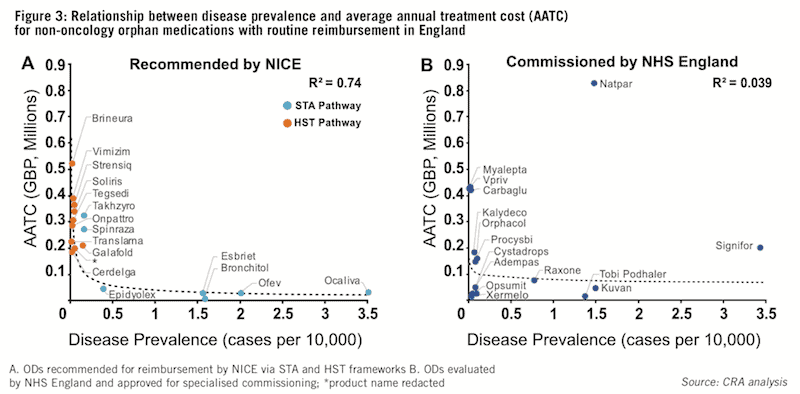
The non-linearity of this relationship can be explained by two key differences in NICE’s STA and HST assessment frameworks: first, the eligibility criteria of the HST framework restricts access to drugs targeting ‘very rare’ diseases (typically ultra-orphan conditions with a prevalence of <1:50,000), and second, the difference in willingness to pay and incremental cost-effectiveness ratio (ICER) thresholds.
The STA framework’s ICER threshold is £20,000-30,000 per incremental quality-adjusted life year (QALY) gained whereas the HST framework’s ICER threshold is based on a sliding scale, beginning at £100,000, with the possibility of reaching up to £300,000, per incremental QALY gained. ODs assessed via the HST pathway are thus not only restricted to diseases with very low prevalence but are also able to access a greater ICER threshold.
This helps explain the non-linearity of the relationship between disease prevalence and AATC for ODs assessed by NICE. In contrast, there appears to be no relationship between disease prevalence and AATC for ODs evaluated by NHS England for specialised commissioning (see Figure 3B).
We believe this is due to the lack of formal incorporation of the value of disease rarity within the specialised commissioning framework, which weighs heavily on the clinical effectiveness and budget impact of new medicines.
There are no stipulations within the framework that allow for higher costs based on disease rarity and, conversely, medicines that are either cost saving or cost neutral are subject to fewer assessment steps compared to those that require additional investment. Findings also indicate that ODs recommended for reimbursement by NICE are typically reimbursed at a higher AATC than those reimbursed by NHS England, with a median of £220,256 (NICE) versus £147,156 (NHS).
Overall, disease rarity is differentially incorporated into reimbursement decision-making in England. NICE formally incorporates the value of disease rarity in its HTAs by increasing the willingness to pay threshold for ‘ultra-rare’ diseases while there is no formal assignment of value to disease rarity in assessments conducted by NHS England.
Are drugs for rare diseases disproportionately subject to commercial agreements?
We also aimed to better understand the level and type of commercial agreements ODs are subject to in England given that decision-makers would
use commercial agreements for ODs to manage the uncertainty that is often associated with their clinical trials and data. Our analysis focused on ODs recommended for reimbursement by NICE as any commercial agreements are clearly stated in their guidance; there is less transparency on commercial agreements for ODs commissioned by NHS England.
The results show commercial agreements are common, with 88% (15/17) of the ODs reviewed in the study subject to at least one type of commercial agreement (see Figure 4). Unsurprisingly, due to decision-makers’ preference for simple solutions, the type of agreements NICE uses most frequently are simple discount patient access schemes (PASs).
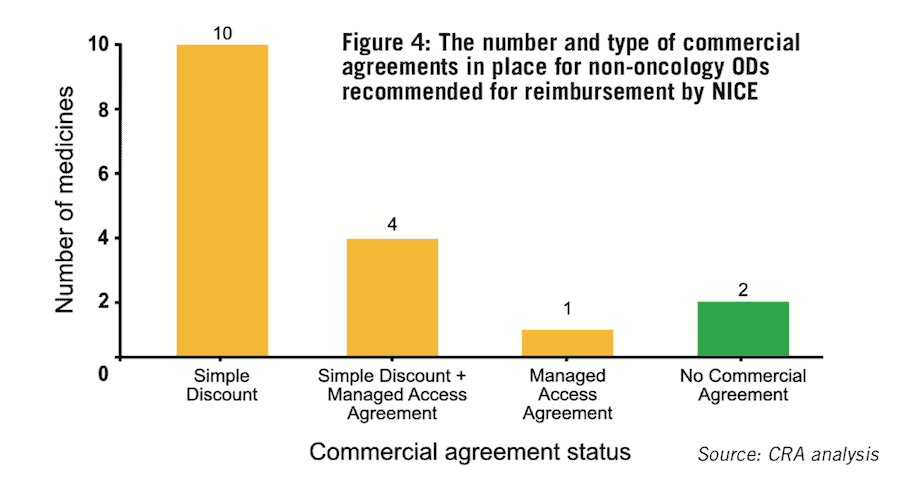
These confidential discounts were agreed for 82% of reimbursed drugs (14/17) in the study (see Figure 4), which is double the figure reported from a recent analysis of 313 medicines appraised via NICE’s STA pathway (41.5%).
Confidential discounts can be sizeable and can significantly impact the net price; however, the ability to engage in such agreements is often driven by situations where manufacturers face a choice between a high discount or no reimbursement. Commercial agreements also offer the advantage of being able to maintain the list price, which can be useful to manufacturers for subsequent launches in markets using England for external price referencing.
The other type of commercial agreements NICE typically uses is managed access agreements (MAAs), which can take various forms and involve different factors including access to specific patient subpopulations only, starting/stopping rules, collection of real-world evidence and continuation
of funding being dependent on clinical outcomes.
They offer the opportunity to gain market access in cases where there is a significant level of uncertainty in the clinical data of ODs and they allow for the ongoing collection of data to strengthen the evidence base and secure permanent or wider reimbursement. These agreements are used to a lesser degree for ODs recommended for reimbursement by NICE, with about 29% (5/17) of the ODs in our data set being subject to an MAA. This is perhaps as a result of the complexity and investment required for their implementation.
Conclusions
While NICE often bears the brunt of criticism for a lack of holistic decision-making, it clearly captures the value of disease rarity more formally than NHS England. ODs assessed through NICE are more often recommended, and typically at a higher AATC, compared with those assessed by NHS England, although this could be at the cost of net level discounts and/or conditional agreements.
Our findings suggest that manufacturers of ODs need to carefully consider their available routes to reimbursement in England as their choice may impact both market access and AATC. Manufacturers of ODs should aim to use NICE’s HST pathway where possible, as it confers the greatest opportunity to capture the value of disease rarity.
In cases where ODs will not meet NICE’s cost-effectiveness thresholds, manufacturers may still be able to achieve reimbursement via NHS England specialised commissioning. They will also need to monitor how their options may change over the coming years, given that since April 2020, NICE is expected to appraise all new active substance unless there is “clear rationale not to do so.”
The views expressed herein are the authors’ and not those of Charles River Associates (CRA) or any of the organisations with which the authors are affiliated.




