
In January, as the (then unnamed) novel coronavirus started spreading across the globe, media coverage in the Western world urged people not to panic, reminding them that ‘regular’ flu posed a greater threat to their health.
Now we find ourselves in October, over six months since the first lockdown, and with many countries now undergoing a ‘second wave’ of infections. With the benefit of hindsight, it might seem easy to scoff at the following quote from Dr William Schaffner, a professor of preventive medicine and health policy at Vanderbilt University Medical Center in Tennessee.
“When we think about the relative danger of this new coronavirus and influenza, there’s just no comparison. Coronavirus will be a blip on the horizon in comparison. The risk is trivial.”
While the drastic public health measures taken to combat COVID-19 are devastatingly far from a ‘trivial blip on the horizon’, with over one million officially recorded deaths from the virus worldwide, Dr Schaffner had a point to call attention to the dangers of influenza.
Influenza is deadly – in fact, from January to mid-April this year, three times more people in the UK died from flu or pneumonia than from COVID-19. Vaccines have been available to protect against both influenza and pneumococcal disease for many years now, but demand for vaccination against these conditions has never captured the public’s imagination in the way it has with the demand for new vaccines to combat the current pandemic.
Suboptimal uptake of vaccines included in immunisation programmes has long been recognised as a public health challenge, with policymakers and manufacturers alike attempting to develop solutions to tackle barriers across
the ‘5As’ dimensions of Access, Affordability, Awareness, Acceptance and Activation.
The question now is whether COVID-19 will help or hinder the quest to increase uptake of other vaccinations, considering the potential opportunities, challenges and threats.
Opportunities – time to tap in to policymakers’ drive for prevention and leverage the public’s increased attention on public health
COVID-19 has certainly brought public health back into the spotlight. The rise in ‘armchair epidemiologists’, spreading their views on how to ‘reduce the R rate’ and ‘flatten the curve’ across social media, has been a parallel epidemic of its own. We are all feeling personally affected by the crisis, and this has been reflected in increasing familiarity amongst the general public with new concepts – including the value of vaccination and the pros and cons of different technologies and approaches.
‘All publicity is good publicity’, as the saying goes. So with more people than ever talking about the value of vaccines in the context of COVID-19, it doesn’t seem unreasonable to expect that we might see a ‘halo effect’ on vaccinations more generally, as people become more educated regarding the burden of other diseases.
As with the increased attention on other public health measures such as handwashing during these times, one might speculate that people will be more motivated to seek out vaccinations because of their greater awareness and fear of COVID-19.
Public health officials and policymakers are pushing this too. The burden of COVID-19 on the healthcare system is driving a need to find ways to help relieve the pressure. Increasing vaccination coverage rates against other preventable diseases is, in the scheme of things, a low-cost way to achieve this, particularly for diseases that affect similarly vulnerable populations, such as flu and pneumococcus.
Avoiding a dual disease burden or co-epidemic with COVID-19 is a priority for hospitals suffering from already strained resources and limited inpatient capacity. Although influenza and SARS-CoV-2 are unrelated infections, some scientists have even speculated there is a possible cross-protection, or adjuvant effect, of influenza vaccination on reducing the severity of COVID-19.
There is also the likelihood that healthy people are better able to defend against the virus; a Brazilian study of over 90,000 confirmed cases of COVID-19 published in June found that patients who had received an influenza vaccine were 8% less likely to need intensive care treatment and 17% less likely to die.
As the northern hemisphere braces itself for a second wave of SARS-CoV-2 infections that looks set to coincide with the impending flu season, manufacturers of flu vaccines have been gearing up to meet the increased demand.
There is still a long way to go to improve coverage rates, particularly when it comes to seasonal influenza. European health ministers committed to achieve 75% flu vaccination among over 65s back in 2009 – but each year only 44% are actually covered, with not one country hitting its target.
As the pandemic hit, some countries made early moves to extend their seasonal influenza vaccination programmes to include other vulnerable populations. Ireland proposed extending vaccination to all children aged 2 to 17, while Belgium, one of the countries hit hardest in terms of COVID-19 death rates, recommended vaccinating everyone between the ages of 50 and 65.
In England, the health system is working to provide a free flu vaccine to 30 million people, the highest number on record. All primary school children and, for the first time, Year 7 children will be offered the flu ‘nasal spray’. Two- and three- year-olds will be offered the vaccine.
As usual, the most vulnerable, including adults aged 65 and over, those with long-term health conditions and pregnant women, will be offered a free flu vaccine. It will also be offered to household contacts of people on the NHS Shielded Patient List and all health and all social care workers who have direct contact with the people they care for.
A newly eligible group comprising 50- to 64-year-olds will also be offered a free flu vaccine later in the season. Anyone who is 50 to 64 years old with long-term health conditions should be vaccinated earlier in the season, in line with all others in risk groups.
Interim guidance published by the WHO Strategic Advisory Group of Experts (SAGE) on 21 September suggests that there is a need to reconsider the prioritisation of risk groups for influenza vaccination during the COVID-19 pandemic.
Even though influenza infections in the southern hemisphere were actually dramatically reduced during this year’s flu season due to non- pharmaceutical interventions intended to limit the spread of COVID-19, such as hand hygiene and restrictions on population movement, as travel restrictions start to be lifted we can expect to see increased transmission.
As such, policymakers are continuing to stress the importance of planning for seasonal influenza circulation this winter.
Challenges – overcoming the logistics of vaccination during lockdown, and managing supply shortages
While the desire to increase vaccination coverage rates is all well and good, two major barriers stand in the way of realising this objective, at least in the short term. The first is actually administering vaccines during these socially distant times, the second is securing sufficient supply once we emerge from them.
Although people might be motivated to take more public health measures out of fear, fear may also translate into a reluctance to present to clinics for vaccination due to the risk of potentially exposing themselves to infection.
For those who are shielding from COVID-19 and thus not coming into close contact with other people anyway, their risk of exposure to other diseases, and the need to be vaccinated against them, is arguably lower – as was borne out by the historically low levels of influenza activity encountered in the southern hemisphere during the June to August flu season.
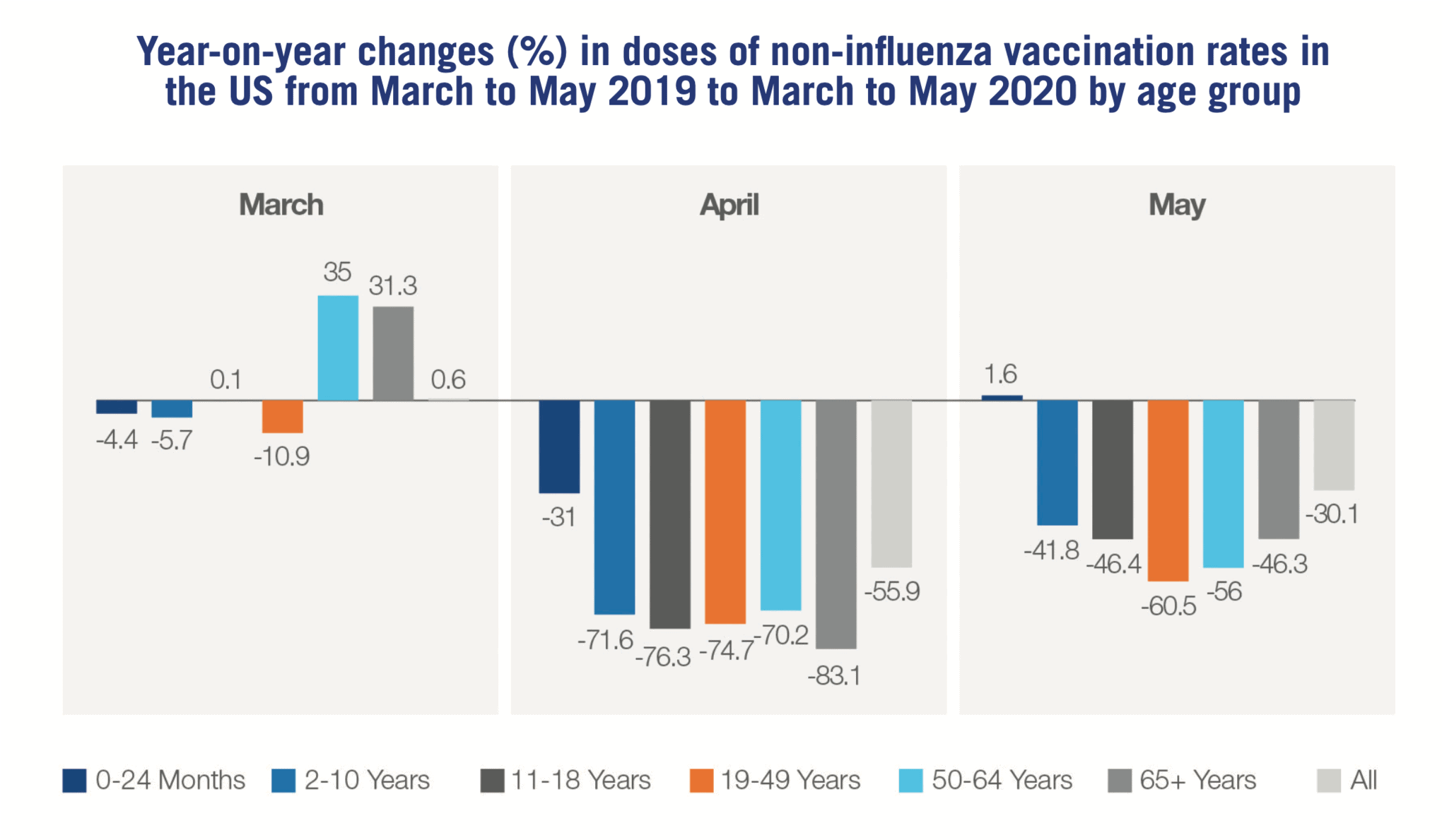
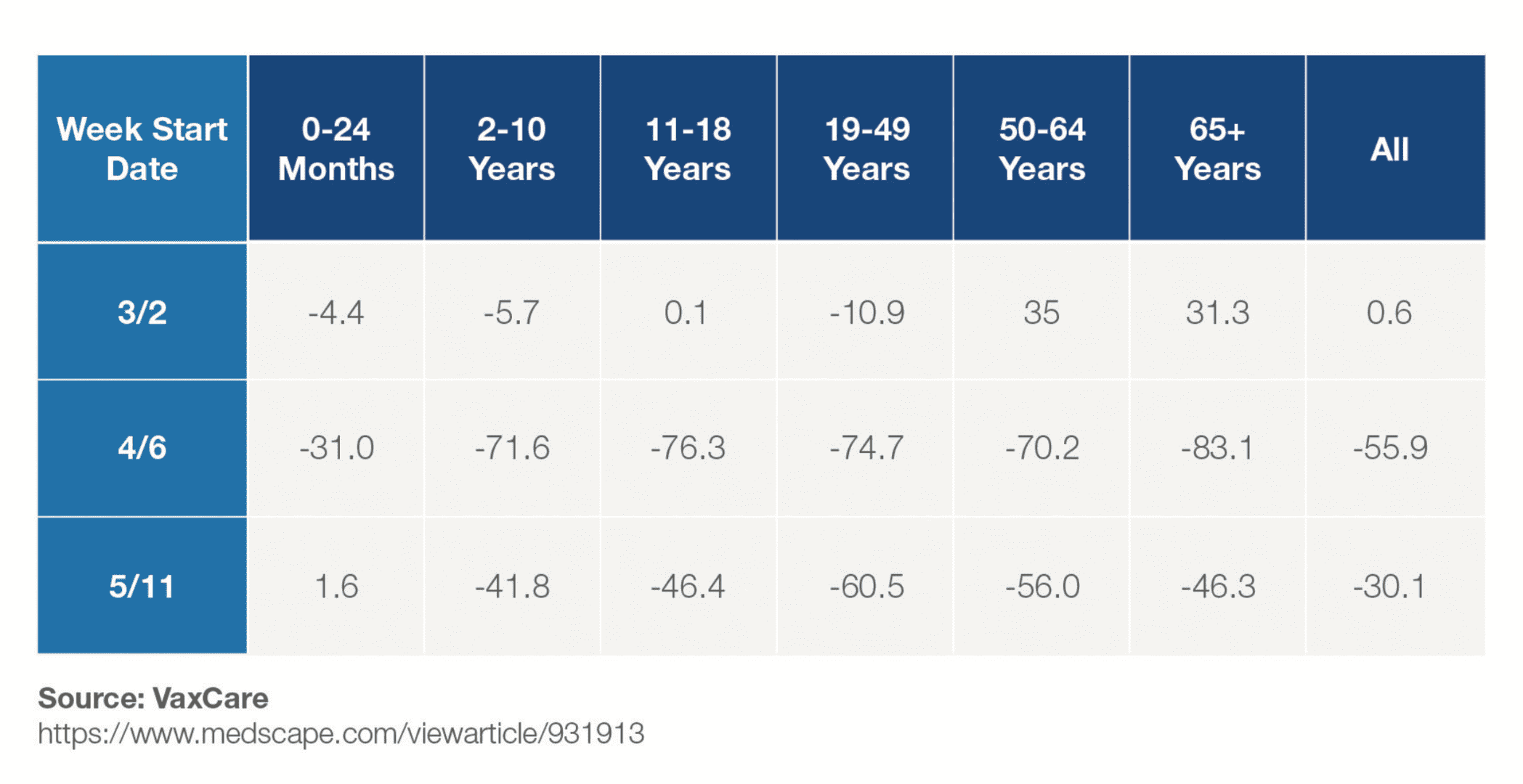
Enforcement of restrictions on movement and postponement of some immunisation programmes have, in some areas, taken this choice out of their hands. In the US, data released earlier this year shows a dramatic drop in vaccination rates across all age groups during the first months of COVID-19 lockdowns.
As restrictions began to be lifted in the summer months, the prospect of reopening institutions, schools in particular, ignited a debate around the risk of missed immunisations during the lockdown. Some argued the backlog of postponed shots would need to be dealt with before schools could reopen safely.
With catch-up doses adding to ongoing demand, the issue of supply needs to be considered. Flight cancellations, trade restrictions and border closures have all impacted supply chains and production timelines, constraining global access to vaccines.
Shortages will take time to address, as manufacturers often require months of lead time to ramp up production in response to increased demand – not all are able to keep up. BCG, widely used for childhood immunisation against tuberculosis, has been particularly affected due to experimental use as a treatment for COVID-19.
These challenges look set to become even more acute in low and middle income countries (LMICs). At the end of April, Gavi, the Vaccine Alliance, was already reporting vaccine shortages in at least 21 LMICs. With already limited financial resources diverted to COVID-19 response, immunisation services in LMICs are facing considerable disruptions, from which their less resilient infrastructure may take longer to bounce back.
Robin Nandy, Principal Advisor & Chief of Immunizations at UNICEF, recently used the example of Ebola to caution against the dangers of secondary impacts on public health if planned vaccination delivery is neglected in the face of the current pandemic, saying, “Twice as many children died of measles than of Ebola, during the last Ebola outbreak.”
It is clear that the industry and the public health community will need to work together even more closely to maintain essential vaccination supply and coverage through these challenging times.
Threats – what if a rushed SARS-CoV-2 vaccine fuels anti-vaxxer arguments and erodes public trust in vaccination?
Thinking back to the ‘5As’, a particular threat to mass vaccination programmes in recent years has related to Acceptance – the rise in vaccine hesitancy, with ‘anti-vaxxer’ beliefs spreading across social media like wildfire. One might think the positive PR around vaccinations resulting from COVID-19 alluded to earlier might partially combat this. However, conspiracy theorists are attracted
to pandemics like moths to a flame, allowing dangerous potential for ‘fake news’ to flourish unchecked. A May 2020 poll of the UK public revealed the alarming statistic that one in eight believe the current pandemic is part of a global effort to force everyone to be vaccinated.
Herd immunity, the resistance to the spread of a contagious disease within a population that results from a sufficiently high proportion of individuals being immune to the disease, is a key concept in vaccination.
Scientists have suggested that between 55% and 82% of the population would need to be vaccinated against SARS-CoV-2 to meet the minimum threshold for herd immunity – so achieving high coverage rates would be critical to protect anyone ineligible for the vaccine.
As seen with seasonal influenza vaccines, achieving coverage rates above 50% of even the over 65 population is already a major challenge; vaccinating such a high proportion of the population across age groups will potentially
be even more difficult. The success of any programme could therefore be undermined by the efforts of anti-vaxxers if their views gather sufficient momentum.
A particular threat in this regard could come from the accelerated clinical development programmes and expedited approval processes that are being put in place to push through SARS-CoV-2 vaccines.
Stewart Lyman, a biotech and pharma consultant, recently cautioned: “Coming up with and launching a vaccine fast – but one that is neither safe or efficacious, or is just one of those – would be an unmitigated disaster. Not just for the COVID-19 programmes, but for every other vaccine made these days. Because then you’re going to get people who are afraid, pushed by these anti-vaccine groups, and starting to question the safety and efficacy of other vaccines.”
It is therefore critical that minimum standards for safety and efficacy are maintained, protected and closely assessed; corners cannot be cut. Recent controversies surrounding the release of data on promising COVID-19 therapies in press releases prior to publication of detailed trial results, and the retraction by the Lancet of the paper that halted hydroxychloroquine trials due to data inconsistencies, if repeated in the context of SARS-CoV-2 vaccines, risk undermining public confidence in vaccines.
Notably, Russia’s Sputnik V vaccine, developed by Moscow’s Gamaleya research centre, and announced by President Vladimir Putin in August as being the world’s first registered coronavirus vaccine, despite no large-scale clinical trials having been completed, caused widespread concern among the scientific community. Natalie Dean, assistant professor of biostatistics at the University of Florida, cautioned in August that “a weak vaccine might be worse than no vaccine at all”.
Conclusions – a golden window of opportunity for manufacturers to act (with care)
With the world paying more attention to public health and vaccination than ever before, the time is ripe for vaccine manufacturers to capitalise on this by demonstrating the public health value of their products in the context of this pandemic to policymakers, prescribers and the public.
To date, much of the media focus has been on seasonal influenza vaccines, but with prevention a hot topic more broadly, there is also the potential for manufacturers of vaccines against other diseases to tap in to this (unless, of course, they are focused on the globally slumping travel health market…).
Pneumococcal vaccines, in particular, could also be positioned as helping to avoid the dual burden of other respiratory infections and thus relieve pressure on healthcare systems strained by COVID-19. However, it won’t all be plain sailing for vaccine manufacturers who wish to navigate their way successfully through this crisis.
Translating positive messaging into increased sales will require tight management of supply chain logistics, and working with the public health community to work against anti-vaccination messaging, as well as ensuring scientific integrity and close scrutiny of evidence generated for SARS-CoV-2 vaccines. Not only to safeguard those who receive these vaccines, but also to avoid compromising the reputation of the entire industry.
Throughout, a detailed and updated understanding of how COVID-19 is affecting the ‘5As’ dimensions of Access, Affordability, Awareness, Acceptance and Activation will be critical for manufacturers looking to understand where to play and how to win for their vaccines in a post-COVID world.
Visit www.researchpartnership.com/vaccines for more information on Research Partnership’s experience and expertise in vaccines market research.





