Today Vertex is synonymous with its groundbreaking cystic fibrosis drugs (and also for pricing and access controversies), but targeting the cruel, genetically inherited disease wasn’t part of the vision of its founders Joshua Boger and Kevin Kinsella in 1989.
Ex-Merck & Co. scientist Joshua Boger did want the company to pioneer rationale drug discovery and development, however, and after
a decade of trial and error, the company had a huge breakthrough drug on its hands: hepatitis C treatment Incivek.
Launched in 2011, Incivek enjoyed the most successful launch in pharma history, earning $1.6bn in sales during its first 12 months on
the market. But when Gilead’s faster-acting and more effective treatment Sovaldi arrived at the end of 2013, Vertex’s drug went from hero to zero overnight. Vertex shares fell 50% in six months, and it was clear the company had to pursue a plan B.
Luckily, that plan B was waiting in the wings: Kalydeco (ivacaftor), the first ever disease-modifying treatment for cystic fibrosis gained approval in 2012.
As many commentators have pointed out, early research into the disease was only sustained thanks to funding of around $200m from US charity, the Cystic Fibrosis Foundation. However Vertex has invested a total of $11.8bn in R&D since 2000, with 70% of its operating expenses reinvested into research over a five-year period.
Speaking about its gamble on CF, CEO Jeff Leiden said: “When we started in CF and said we were going to make a medicine that could actually fix this misfolded, mutated protein in patients, other scientists laughed at us, they didn’t think it was possible, yet here we are 15 years later and today we are treating the disease in almost half the patients around the world.”

Jeff Leiden, Vertex CEO
The company built on its initial ‘precision medicine’ Kalydeco, which helps just 5% of patients with the specific mutation called G551D, adding Orkambi (ivacaftor/lumacaftor), which treats the F508del mutation and 50%
of CF patients and now Symkevi (ivacaftor and tezacaftor). This is effective for people who have two copies of the F508del mutation plus those with one of 14 other mutations.
This growing portfolio helped Vertex record revenues of $3bn in 2018, and registering a profit of just over $2bn. This certainly looks like a very healthy business, but as Jeff Leiden was at pains to point out to the recent health select committee hearing in London, the company has made a loss in almost all of its 20+ years, and has cumulative net losses of around $3bn.
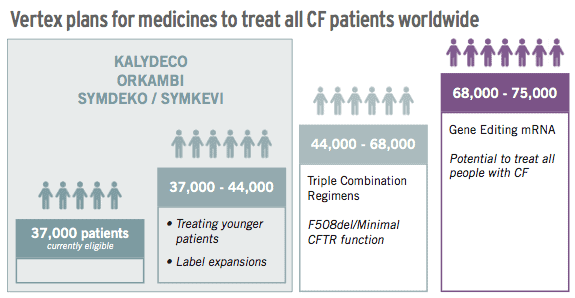
All that means is that 2019 and beyond are the years in which the patience of Vertex shareholders will be paid off. That’s because Vertex now looks set to achieve its vision of being able to treat 90% of the world’s 75,000 CF sufferers within the next decade, a hugely significant achievement in medicine.
This achievement will be capped off if the company can achieve the ultimate goal of developing a gene therapy that could cure all CF patients in a single treatment.
While this final goal remains only a possibility today, the company is on course to reach up to 68,000 patients worldwide with its forthcoming triple therapy.
In late March, Vertex was boosted further when its main challenger in the field, Proteostatis Therapeutics, reported disappointing results for its rival triple therapy candidate.
This adds to the good news for Vertex, which saw AbbVie and Galapagos also post disappointing results for their triple therapy candidate.
This gives Vertex a clear run at being the first to market and best in class for the next five years at least. Vertex is running two parallel triplet studies, VX- 659 + Symdeko and VX-445 + Symdeko, with one expected to emerge as superior, and be selected for filing.
One study was carried out in CF patients with one F508del mutation and one minimal function mutation, with a 14% improvement over placebo, while the other involved patients with two F508del mutations and showed a 10% improvement.
The chosen candidate is then expected to gain approval in the US market in the first half of 2020, followed by Europe in the first half of 2021.
The failure of Proteostasis has led analysts to upgrade forecasts for Vertex’s own franchise.
Y. Katherine Xu, an analyst at William Blair, forecast peak annual sales of $10.2bn, up from $8.9bn previously, representing a market share in the US and EU of around 80-85%. The analysts also now predict Vertex will be able to maintain its prices in both markets, rather than have to drop them slightly in the face of competition.
Vertex set for dominance
The failure of Vertex’s rivals will come as a disappointment to healthcare payers in the US and Europe, who had hoped for competitors to help drive the company’s prices down.
The UK is one country where it has met concerted resistance to its prices. After its grilling of Jeff Leiden and his NHSE and NICE counterparts, the chair of the health select committee, Dr Sarah Wollaston MP wrote to the health secretary suggesting the government could refer the company to the Competition and Markets Authority for “what appears to us to be the exploitation of a monopoly position in the supply of drugs for the treatment of cystic fibrosis”.
This looks like an unlikely scenario, although Vertex’s prices will remain a source of friction over the coming years in a lot of markets worldwide.
A gene therapy cure for all the world’s CF patients would be a truly remarkable achievement. However, as in other disease areas, it threatens to upend the biopharma business model: once you’ve cured the patient with a singe treatment (even at $500,000 or more) you have also cut off your long-term revenue stream.
That’s why Vertex is now looking beyond CF to other orphan diseases to conquer. These include alpha-1 antitrypsin deficiency and focal segmental glomerulosclerosis.
It’s also working with CRISPR Therapeutics on phase 1/2 clinical trials for CTX001, a gene editing therapy for patients with sickle cell disease or beta thalassemia with FDA Fast Track Designation. While it’s understandable that Vertex has been seen as ‘evil biopharma’ for its pricing strategy, it has also been lauded for its truly groundbreaking innovation, and for taking bigger financial risks than many of its industry peers.
As this gamble starts to pay off, it’s no surprise that it is also frequently named among the most likely M&A targets in the industry. It’s yet to
be seen if Vertex will get gobbled up by a big biopharma company, or if it turns into one itself. What’s for sure is that it’s already an exemplar of what a small, science-led company can achieve over the span of two decades.