
The recent approval of Hospira’s Inflectra (infliximab) may mark a turning point for biosimilars in Europe. While follow-on biologics have been on the EU market since 2006, Inflectra was the first such approval for a monoclonal antibody (mAb) – larger, more complex molecules than the 14 biosimilars previously cleared by the European Medicines Agency (EMA). The broader context is that the exhaustion of patent and other intellectual-property rights on originator biologics over the next decade affords an unparalleled opportunity for biosimilars to enter the market and boost competition in the sector.
Here we consider how the European landscape for biosimilars is ripe for transformation as more mAbs or anti-TNFs (Tumour Necrosis Factors) for inflammatory conditions such as rheumatoid arthritis enter the fray.
First of all, there needs to be genuine uptake in Europe. And that requires buy-in from specialists, doctors, payers and patients. Although Europe remains well ahead of the US in mapping out a viable approval pathway for biosimilars, the market impact to date has been somewhat muted.
With a persistent hum of unease around comparability, quality, therapeutic equivalence and immunogenicity, and with generic substitution all but off the menu, stakeholder awareness and confidence will be key drivers of future revenues and savings in the category.
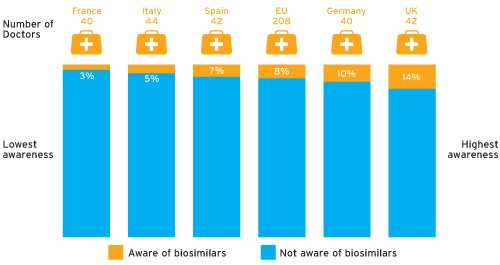
Yet awareness of biosimilars among physicians is currently very low. According to data supplied by Therapy Watch, just 8 per cent of physicians questioned in the EU spontaneously mentioned biosimilars as products in development (see chart overleaf), with Remsima/Inflectra the only biosimilar mentioned specifically by name.
It is clear that companies entering the European market will need to pay close attention to education, awareness, confidence-building and physician/patient support. They must also address concerns about safety, efficacy and manufacturing quality – whether or not those concerns are justified.
Seeking the opinion of a European physician, Dr Anissa Hacène, a member of the Therapy Watch panel from France, told us: “Currently we don’t have enough trial data or hindsight on these drugs to demonstrate equivalent efficacy and similar levels of side-effects. If they were backed by trustworthy, large-scale clinical studies, we could probably think about this differently.”
Biosimilars are on the agenda at congresses and in medical journals, but “we have never received a presentation about them and we haven’t had anyone come to talk to us about them in an official capacity”, she adds. “I think there is currently little information out there to defend their use, whereas there is a lot that encourages us to be suspicious about them.”
The Therapy Watch data tells a similar story. For example, only 35 per cent of the panel said they would definitely consider prescribing biosimilars for rheumatoid arthritis (RA) within one year of launch.
The most positive response, not surprisingly, came from Germany – regarded as the most biosimilar-friendly market in Europe. Here, 58 per cent of physicians would consider prescribing biosimilars within a year of launch.
Manufacturers of biosimilars will also need to deliver a value story that is about more than just price
Physicians who said they would not consider launching biosimilars within a year of launch mostly cited concerns about purity versus the originator product, immunogenicity and efficacy/effectiveness. One London-based budget-holder, Dr Anthony Grosso, who is principal pharmacist, and honorary associate professor in clinical pharmacy practice, at University College London Hospitals, sees a real lack of knowledge and understanding around biosimilars, particularly in relation to quality and pre-market testing. Developing and manufacturing a biosimilar is “not a tinpot operation”, he insists.
Pricing will be another decisive factor. Cost savings from available biosimilars in Europe have so far been relatively modest, with reported discounts of 10-30 per cent on reference brands. Budget-holders will be looking for “quite a considerable differential”, Dr Grosso also warns. The “only reason to use biosimilars really is that they’re going to be less expensive … we’re expecting about 30 per cent. Anything less would be disappointing”. This feeling is echoed by Dr Hacène. She currently estimates that biosimilars are only 15-20 per cent cheaper than their originators but states that a 50 per cent discount would be even more interesting.
Which products in development, if any, are you aware of for the treatment of RA? (Q2 2013 by country)
At the same time, though, the biosimilars industry will need to bear in mind the possibility that originators may lower their own prices to drive uptake by physicians, patients and payers, leading to unsustainable price erosion.
However, Dr Hacène advised caution. “At that point you would have to question whether a drug that can be manufactured for 50 per cent less is as pure and isn’t more likely to create allergic reactions.”
So, manufacturers of biosimilars will also need to deliver a value story that is about more than just price. Finding early adopters to act as key opinion leaders (KOLs) for these products will be critical. KOLs will want reassurance over practical issues such as supply-chain logistics, particularly if biosimilars are manufactured abroad. Manufacturers will also have to compete against value-added services offered for some biologics, such as healthcare at home.
Physicians will certainly play an important role in future uptake of biosimilars. As the Therapy Watch data indicates, though, many believe the decision will ultimately be taken out of their hands. Of the physicians questioned, 66 per cent agreed their use of biosimilars would be dictated by local or national guidelines/protocols (UK highest with 77 per cent, Germany with 49 per cent).
“If regional and/or national recommendations support the use of biosimilars, then we will need to follow them – as long as they remain reasonable, of course,” Dr Hacène comments. “For example, if they were to suggest that everyone should be treated with biosimilars, I think physicians would defend their existing treatment decisions and refuse to switch patients.” Dr Grosso adds: “I don’t think this is going to be a clinician-driven thing at all. Rather the drive for usage will come from the local or regional level.”
With research-based multinationals taking a growing interest in the market, access strategies may progressively rely on distinguishing between high-end biosimilars – those with resources to back up product claims with additional data – and lower-cost biosimilars developed in emerging markets.
But marketing platforms at all levels must drive home the message that biosimilars are a very different proposition from commodity generics.
Some companies have “done a really bad job of launching their biosimilars”, Dr Grosso comments. “They’ve just thrown them into the market at a good price, expecting uptake.” Instead, he insists, companies with biosimilars “really need to treat them as if they were branded medicines, and with the full sales and marketing that goes with it”.
There is little doubt that biosimilar medicines present a significant opportunity to embrace cutting-edge therapies while addressing the cost-effectiveness demands on healthcare systems across Europe – and perhaps none more so than in a chronic condition associated with rapidly ageing populations, such as rheumatoid arthritis.
So Inflectra could prove to be not just a regulatory breakthrough but a potential game-changer for the whole biosimilars class, one that companies waiting for a clear pathway in the US will be watching very carefully.
All the same, achieving widespread acceptance and uptake of biosimilars in Europe is by no means a done deal. Companies entering the market will need to ensure they have in-depth customer insights to offer a real value proposition that makes a meaningful difference to patients, healthcare professionals and increasingly hard-pressed healthcare systems.
To request the full white paper email contact@researchpartnership.com





