Executive summary
- One of the Asian Tiger economies, South Korea has maintained rapid growth levels, low state debt and has shown resilience to financial crises due to strong exports
- Considered one of the most ‘wired’ nations in the world, with almost universal access to the internet, South Korea excels in technology goods
- The implementation of the Free Trade Agreement with the US in March 2012 will have a considerable impact on the healthcare market and particularly on the domestic industry
- The business environment has improved with the internationalisation of the country
- The healthcare system is high quality, universal and based on choice
- Seoul as a medical tourism destination is booming, with strong government support, through measures such as allowing prescription of medicines to foreign patients
- Since 2006, cost-effectiveness has been top priority for policy makers with the ‘Rationalisation plan’
- Although healthcare spending in general is low compared to other OECD nations, pharma expenditure is around 24 per cent of the total national health insurance system’s bill
- The South Korean pharmaceutical market has had consistent double-digit growth for five years and is considered the fastest-growing developed country by IMS Health
- Aggressive price cuts on branded medicines and generics and delisting of reimbursable products have slowed pharma growth
- Lack of predictability and transparency in pricing and reimbursement decisions has concerned multinationals and trading partners in the US and European Union
- Generics face stricter patent protection, price cuts and competition, particularly from India and China. It is a crowded market, with low growth forecast in the current climate
- The price differential between generics and originators is negligible and doctors and patients prefer branded products
- The biotech industry is thriving, with over 20 bioclusters and government investment and tax breaks
- The outlook is uncertain, although due to the size and strength of the market, Korea will remain attractive to investors, particularly those looking to pursue innovation and partnerships with local manufacturers.
|
Overview Name: The Republic of Korea |
Introduction
South Korea is the fourth largest economy in Asia and thirteenth in the world by GDP. It was a Chinese tributary state for centuries until it became independent in 1895. It was annexed by Japan in 1910, regaining its independence after World War II, which led to partition with a democratic government in the southern part of the country and a communist-style state in the north.
Under the military rule of the Park Chung-hee regime, South Korea achieved rapid economic growth and in 1993 it elected its first civilian president, Kim Young-sam.
For many decades government-sponsored schemes encouraged the growth of family-owned industrial groups, known as ‘chaebol’. These have helped to turn South Korea into one of the world’s strongest economies and exporter of cars, automotive parts and high technology goods.
North Korea’s nuclear aspirations and economic turmoil have threatened Seoul’s stability. Since the Korean War finished in 1953 with no treaty, the two countries have been embroiled in conflict, although since the 1990s South Korea’s strategy has been to resist backing international sanctions, opting for ‘sunshine engagement’, involving aid, tourism initiatives, contacts across the border and economic cooperation.
Since he took office in 2008, Conservative President Lee Myung-bak has pursued a policy of global engagement and a harder line regarding North Korea, particularly after Pyongyang’s missile tests in 2009. Relations have further deteriorated with the North’s sinking of the South Korean warship Cheonan and attacks on South Korean soldiers and civilians in 2010. The current President has served a five-year term and will step down in December.
South Korea’s structural stability is threatened by the ongoing conflict with North Korea. Seoul is watching its secretive neighbour closely, whose leader Kim Jong-il died in December 2011, being
replaced by his third son. Some analysts consider reunification a real prospect, with massive economic and social implications, but there is no indication such seismic political change will take place in the near future.
Economy
Following the Asian financial crisis of 1997-98, Seoul adopted several economic reforms, including increased openness to foreign investment and imports. South Korean banks have been exposed to high levels of foreign debt and felt the impact of the latest global financial crisis, with growth slowing to 0.2 per cent in 2009.
However it was one of the few developed nations to avoid a fullblown recession. On the back of strong exports and low interest rates, the economy started to grow, reaching an estimated 3.9 per
cent in 2011.
One of the country’s main challenges is the rapidly ageing population, coupled with an inflexible labour market and overreliance on exports, which comprise half of GDP. After the 2007 crisis, South Korea ran external deficits, due to exports tumbling and oil prices soaring, but it has now returned to sustainable trade surpluses.
Domestically focused small and medium sized businesses are struggling, as government policies have traditionally favoured the chaebol giants.
IHS Global Insight’s director of Sovereign Risk Jan Randolph told PME: “The weakening world economy hurt Korean growth and will continue to do so in the near term. Exports will soon rebound, thanks to Korea’s highly competitive manufactured goods, but their growth will be slow. Also, domestic spending will be sluggish, mirroring the external sector. This generalised weakness will keep growth rates in the low single-digit range until late 2012.”
The US-South Korea Free Trade Agreement (KORUS FTA) has finally been ratified by both governments. This treaty eliminates 95 per cent of each nation’s tariffs on goods within five years and creates new protections for multinational financial services and other companies. It is the US’s first FTA with a major Asian country and has a considerable impact on pharmaceuticals.
A developed country, South Korea still has a volatile currency, the Won, which behaved as the Brazilian Real and the South African Rand during the 2008 crisis, according to the Korea Institute of Finance.
The Won’s tendency to weaken in a harder economic climate helps its export-reliant industries and international organisations such as the World Bank describe Korea as one of the fastest-growing economies alongside the BRIC nations (Brazil, Russia, India and China) and Indonesia.
Healthcare landscape
South Korea has one of the world’s highest suicide rates, having overtaken that of Japan. It has doubled in the past decade to account for 31 in every 100,000 deaths. An Organisation for Economic Cooperation and Development (OECD) report attributes the rise in people taking their own lives to ‘weakening social integration and erosion of the traditional family support base for the elderly’, as well as a fast-changing society. The leading cause of death is cancer, followed by cerebrovascular and heart disease.
Suicide ranks fourth, according to the Ministry of Health and Welfare. The prevalence of smoking is high among men (50 per cent), according to World Health Organisation data.
The most common cancers in Korea are stomach, colorectal, lung and liver in men and thyroid, breast, stomach and colon in women.
The National Cancer Centre was set up in 1996 to coordinate a national programme with the aim of cutting cancer rates by 2015.
The R&D Program for Cancer Control funds research projects by industry, academia and research institutes.
| Health data | |
|---|---|
|
Doctors per 1,000 people |
1.97 |
|
Hospital beds per 1,000 people |
12.28 |
|
Hospital visits per year |
11 |
|
Obesity rate |
3.2 per cent |
Other health indicators are positive, such as a long life expectancy and the lowest obesity rate in the OECD. Unlike other Asian countries, Korea has maintained a traditional healthy diet (low in fat and high in vegetables).
The National Health Promotion Act of 1995 included interventions and initiatives to deal with nutrition related chronic diseases and to prevent obesity. Rising demographic pressures such as ageing and low birth rate and chronic inefficiencies in the healthcare system are forcing the government to continue its intense focus on cost management.
Healthcare system
South Korea has a universal health insurance system that is compulsory and covers employees and their relatives (National Health Insurance, NHI).
The first social insurance service, introduced in 1977, started in companies that had more than 500 employees and gradually extended coverage to smaller sized firms. In 1981, the scheme expanded to cover civil servants, teachers and other state employees and in 1989, the self-employed were also covered.
There is also a long-term care medical insurance programme and the Medical Aid Programme, co-funded by central government and the NHI, which covers 3 to 4 per cent of the population (those on low incomes and, since 2004, patients with rare, intractable and chronic diseases as well as children under the age of 18).
Total health expenditure (% of GDP)
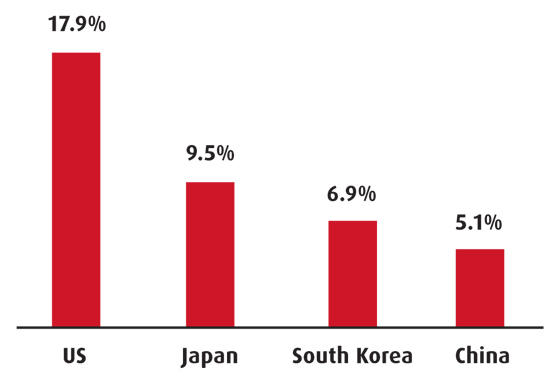
Source: World Bank, most recent
Over the last few decades healthcare costs have risen considerably, although Korea’s expenditure, at 6.8 per cent of GDP, is among the lowest in the OECD. The NHI began running an annual deficit in 1997 and reforms aimed at reducing it involved greater government contributions from general tax revenues, higher premiums, control of fee increases and stricter monitoring of medical fraud claims. Every year the healthcare budget deficit increases despite attempts to curb spending. According to OECD data, doctors’ consultations are particularly high (around 11 times a year) and hospital discharge rates are low, which increases the burden on the system.
Apart from government and employee contributions, the third source of funding of the National Health Service is the surcharge on tobacco, which accounts for 6 per cent of the total annual projected revenue.
In 2000, the healthcare financing reform merged the health insurance societies into a single payer, the National Health Insurance Corporation (NHIC) to improve equality and efficiency.
The Health Insurance Review Agency (HIRA) is in charge of reviewing claims of providers, thus making reimbursement decisions, and the Ministry of Health and Welfare (MOHW) supervises the operation of the NHI programme as a whole.
| Co-payment levels | |
|---|---|
|
Classification |
Per cent of co-payment |
|
In-patient hospital |
10-20% of total treatment |
|
Outpatient |
|
|
Tertiary hospital care |
Per-visit consultation fee +50 per cent of treatment cost |
|
General hospital |
50 per cent of treatment cost + per visit consultation fee |
|
Hospital |
40 per cent of treatment cost + per visit consultation fee |
|
Clinic |
30 per cent of treatment |
|
Pharmacy |
30 per cent of total cost |
Source: Korea’s National Health Insurance Corporation, 2006
In 2000, prescribing and dispensing functions were separated to remove the economic incentive associated with prescription for doctors in the public system.
The government also focused on pharmaceuticals and their impact on public healthcare budgets. Until then, the list of reimbursable drugs was extensive and new drugs were rapidly approved for insurance coverage. Cost-effectiveness of drugs and their budget impacts were rarely taken into account in reimbursement decisions. The use of economic data in reimbursement decisions has since become a feature of the Korean market.
Other cost-containment measures include application of the ‘market-orientated average transaction pricing system’, under which the government gives incentives to hospitals that purchase drugs lower than net price, by obtaining deals and discounts from pharmaceutical companies.
Patients pay a fee for service when accessing clinical services, and medicines. Hospital doctors are paid a fixed salary and may receive bonuses based on their performance.
Medical tourism
Despite universal coverage and the freedom to choose services and practitioners, public satisfaction with the health system is low because of long waiting lists for treatment and high costs.
Treatment is not completely free at the point of delivery with co-payments ranging from 10 to 50 per cent. However, there are no gatekeepers and patients are allowed unrestricted access to doctors, hospitals and specialists.
Around 90 per cent of specialist doctors and healthcare institutions are private. The public sector’s role in provision is limited to basic primary care services delivered through health centres.
According to the Japan Medical Association Journal ( JMAJ), a major problem concerning healthcare resources in South Korea is that of regional disparities.
Due to medical profit maximisation strategies, most private medical facilities are located in urban areas, as are 92.1 per cent of physicians and 90.8 per cent of hospital beds, according to the JMAJ report ‘The South Korean Health Care System’, from 2009.
The government has tried to boost medical tourism and declared it a target to attract a million foreign patients by 2020. The country already welcomed 120,000 in 2011, states the Korea Tourism Organisation. Last year, the Ministry of Health and the Korea Health Industry Development Institute collaborated on a project to improve the country’s medical policy and infrastructure for foreign patients.
The visa system has been improved and drug prescription in hospitals to non-nationals is to be allowed.
The Ministry will also expand the medical workforce by training and recruiting foreign doctors. South Korea wants to establish a credit union for hospitals treating foreign patients and provide partial support with government subsidies.
Cosmetic and dental treatments are the most common with Seoul boasting a ‘beauty belt’ with more than 200 plastic surgery clinics. Most medical tourists come from the US and China.
Pharmaceutical market
The South Korean pharmaceutical market was worth £12.3bn in 2011 and is the fastest-growing in the developed world, according to BMI Healthcare. Sales growth has been in double-digit figures for the past five years and IMS still forecasts a 6.5 per cent compound annual growth rate (CAGR) through 2015. South Korea ranked fifteenth in the IMS global country league of pharma markets.
The Korea Pharmaceutical Manufacturers’ Association (KPMA) represents around 190 R&D companies. The implementation of the Drug Substance Patent Law in 1987 was a milestone for the industry association. In recent years, 65 of its members collectively invested $2bn to upgrade manufacturing plants in compliance with current Good Manufacturing Practice (cGMP) standards. The Fair Competition Committee was established and a Hotline Reporting Centre for code compliance complaints was activated to increase KPMA’s credibility among the international pharma market.
The business environment has greatly improved and South Korea ranked eighth in the world and sixth in the OECD in 2011, according to the World Bank. Recent government measures to streamline regulations and tax provisions have made it a more business-friendly country, including for pharmaceuticals, with multinationals now representing 40 per cent of the market. Giants such as Sanofi, GlaxoSmithKline and Pfizer have dominated the marketplace for the last decade.
The hospital sector is a strong sales generator, with almost 50 per cent of sales for outpatient departments and 32 per cent for inpatients. Exports of Korean pharmaceuticals are also growing with Japan, China, the US and Vietnam the four largest markets for South Korean pharma products.
The consumption of medicines is widespread and patients are keen on high technology procedures and the latest innovative treatments.
Pharmaceutical expenditure in the NHI accounted for almost 24 per cent in 2008, according to OECD data, which is one of the largest when compared to other countries.
Pharma expenditure (per cent of total health expenditure)
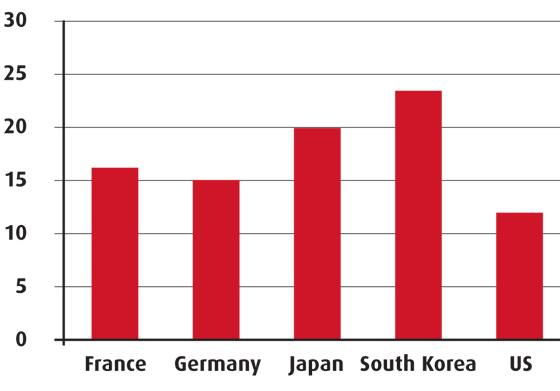
Source: OECD and IRDES, 2010
The government is trying to boost the pharmaceutical market with a fund for R&D, tax deductions for R&D costs, promoting mergers and acquisitions, training workers in the industry and create and sustain a national pharma company to compete on the international stage.
However, Korean companies invest little in developing new drugs, with most making generics, health supplements or traditional Chinese medicine products.
Over-the-counter (OTC) medicines have been a growing segment in South Korea due to a tendency for self-prescription among patients.
Last year, an attempt by the government to allow sales in supermarkets and convenience stores partially failed, as pharmacists have exclusive rights to sell pharmaceutical products, under Korean law.
The Ministry of Health reclassified 44 OTC products as non-drugs to allow sales in stores. There are 21,000 pharmacies in Korea and OTC drugs represent a considerable share of their revenues.
Many experts do not believe that the OTC drug market will be liberalised, as opposition from the pharmacy lobby is too strong.
R&D expenditure (per cent GDP)
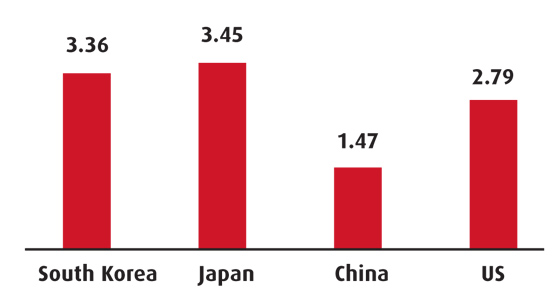
Source: World Bank, most recent
There are significant restrictions on advertising and patients rely on doctors and pharmacists for advice on what OTC medicines to buy. A survey by the Korea Institute for Health and Social Affairs revealed that more than 74 per cent of purchases of cold medicines were made based on pharmacists’ advice, with advertising influencing only 5.6 per cent of purchases.
Advertising of prescription drugs is not allowed and OTC publicity is severely restricted (patient testimonials and ‘before and after‘ comparisons are not permitted). However, drug companies can advertise preventive prescription drugs for infectious diseases in certain cases and sponsor public awareness campaigns, such as anti-smoking initiatives.
US-Korea free trade agreement
The long-delayed US-Korea Free Trade Agreement (KORUS FTA) came into effect in March 2012. The bilateral treatment eliminates 95 per cent of each nation’s tariffs within five years and is the US’s first free trade agreement with a major Asian economy. The Korea Institute for International Economic Policy estimates that general Korean exports to the US will grow by 12 per cent. Seoul already has a FTA with the European Union and is negotiating a similar deal with China.
The Korean government created the ‘2012 Pharmaceutical Industry Competitiveness Enhancement Plan’ to prepare for the KORUS FTA, changing the categorisation of pharma companies into three groups: specialist pharmaceutical firms, global generic companies and major global firms, with differentiated support provided for each group.
It also announced the goals of developing 10 new global drugs, of achieving 5.4 per cent of the global export market share and of creating 12 global companies by the year of 2020.
Tax breaks and loans through the Export-Import Bank of Korea, and the Korea Trade Insurance Corporation are available for innovative companies. Public sector investment in new drug development has been expanded and in training pharma graduates and specialists in licensing and regulatory affairs.
The KORUS FTA strengthens intellectual property protections in Korea, as well as promoting increased transparency in the pricing and reimbursement process. South Korea has been dropped from the US Trade Representative ‘watch list’ due to its efforts to improve patent protection and to strengthen intellectual property enforcements.
However, the Pharmaceutical Research and Manufacturers of America (PhRMA) said in a statement in February 2012 that it was ‘highly concerned’ that provisions regarding the reimbursement of innovative drugs and countering the perceived ‘lack of transparency’ in pharmacoeconomic assessments were not being implemented.
The local pharmaceutical industry is considered vulnerable to the trade pact as it is likely to lose millions of dollars in production every year for the next few years due to delays in the production of generics. The government has set a three-year grace period for local pharmaceutical companies to adjust to the changes.
Patients may also be penalised. Dean Baker, co-director of the Centre for Economic and Policy Research in Washington told PME: “The biggest issue is that the agreement creates a basis for drug companies to contest the government’s pricing policy. They can argue before an independent panel that the prices being set by the government are not consistent with the ‘competitive market price’.
This is a vaguely defined concept; drug prices in the US average more than twice as high as in many other wealthy countries. If the US price is taken as providing any sort of basis for the competitive market price, then people in Korea will be paying much more for their drugs as a result of this trade agreement.”
Pricing and reimbursement
When prescribing and dispensing functions were separated, prescription of branded products soared. However growth has been impaired by a policy of systematic price cuts. Between 15 and 25 per cent price cuts for branded and 33 per cent for generics are planned for this year. More than half of the 14,410 drugs on the reimbursable list are affected. The aim is to save around $1.4bn a year in state spending on pharmaceuticals.
The Drug Expenditure Rationalisation Plan of 2006 initiated a number of reforms, including a system of positive and negative lists.
The Positive List System created in 2008 selected drugs using economic evaluation data. Before that, any medicine that received market approval by the Korea Food and Drug Administration (KFDA) was automatically deemed reimbursable, which meant that the list grew to include over 20,000 products. Korea was the first Asian country to introduce economic evaluations for drug reimbursement decisions.
Price and reimbursement (P&R) decisions have been tightened consequently, following a complex process based on cost-effectiveness.
Access to the positive list and a maximum reimbursement price depends on a favourable decision by HIRA. First the new drug is compared to existing, substitutable ones and a pharmacovigilance study provided by the pharmaceutical company is examined. HIRA then sets the maximum cost-effective price after budget impact analysis for new medicines, indications or additional benefits claimed.
The NHIC opens price negotiations with the drugmaker, taking into consideration the volume of medicines to be purchased and consumed. The single insurer aim is to reduce prices by 20 per cent and these cannot exceed those in main markets such as France, Germany, Italy, Japan, Switzerland, UK and US, though reference countries potentially encompass all OECD ones, plus Singapore and Taiwan. The process can take about a year to 18 months and needs to be ratified by the Ministry of Health and Welfare.
Other mechanisms for lowering prices include evaluations every three years and the recent review of products approved prior to the introduction of the positive list. Lipid-lowering and anti-migraine drugs have been reviewed, resulting in delisting and price cuts. The assessment of hypertension drugs has been delayed but this will be the next class to face price slashes and negative listing.
Concerns have been raised about predictability and transparency in Korea’s P&R system. The European Union Chamber of Commerce in Korea claims that the P&R authorities in Korea do not consider appropriately the value of the new innovative drugs through a ‘consistent and reproducible evaluation of their benefits to the patients as well as their short-, medium- and long-term impact on the healthcare system and the Korean economy and society’.
Generics
Generics represented 15 per cent of the Korean market in 2005, with around 250 companies active. Dong-A, Yoohan, Hanmi, Green Cross and Choongwhe Pharmaceuticals have led in terms of sales.
They are now threatened by aggressive price cuts. Off-patent medicines will be capped at 59.5 to 70 per cent of the pre-expiration price for a year and then 53.55 per cent after that. This is an average 17 per cent price decrease, with some cuts amounting to 33 per cent. Almost half of reimbursable products are subject to cuts.
Four pharma companies, namely Ilsung Pharmaceuticals, Elyson Pharmaceuticals, Dalim BioTech and KMS Pharmaceutical, have asked a court in Seoul to annul the government’s decision to lower prices. Pharma executives and employees have demonstrated in Seoul against the latest price reductions.
Generics companies are particularly affected, with their prices on the same level as, or even below the level of, originator medicines, which are usually preferred by doctors. The Health Minister, Rim Chemin, quoted in the Korea Herald, defended the reforms: “The plan is not a scheme to impoverish drug firms. Koreans’ medical expenses on drugs have been excessive. It needs to change. The National Health Insurance fund is at risk of depletion. If it depletes, the firms will be in danger too. The change is essential for the long run of the industry, too.”
According to IHS Global Insight analyst Aparna Krishnan the government “has repeated its previous insistence that companies would be able to withstand the impact of the price cuts if they put a stop to the still-widespread practice of offering illegal rebates to doctors or hospitals.” IHS Global Insight expects the planned price cuts to adversely affect growth in drug sales and health spending going forward.
Apart from the referred price cuts and the implications of a stricter regulatory environment under the new trade agreement with the US, generic manufacturers also have to contend with increased competition from India and other countries.
In 2009, the government introduced a regulatory pathway for biosimilars. Electronics giant Samsung is investing heavily and strategically in this market.
There is also a strong trend in the development of ‘supergenerics’, or generics brought to market at the same time as patent expiry.
Biotechnology
Biophamaceuticals, including locally-manufactured vaccines, head the list of pharmaceutical items exported (eight out of the top 10 export items are finished biophamaceutical products), according to the KFDA. Investment in biotechnology is increasing and the sector is expected to show an annual growth in double digits in coming years. Strong government support and growing collaboration between the academic and private sectors means that South Korea has become a prime investment location in Asia.
The Korean Government has launched Bio-Vision 2016 to step up competitiveness and improve infrastructure. A previous endeavour, Biotech 2000, failed when chaebols became disenchanted with the lack of profits from biotech and the government withdrew funding. The current policy aims at bioconvergence, integrating disciplines such as biology, bioinformatics and nanotechnology, and creation of bioclusters with the intention of making biotech a thriving sector and lead in international ranking tables.
In the report ‘Knowledge Networks and Nations, global scientific collaboration in the 21st Century’, The Royal Society adds Korea to the list of ‘rising powers in science’, with China, India and Brazil.
According to the association of biotechnology companies, Korea Bio, Korean scientists in biotech have rapidly increased the contribution of research papers, particularly to Nature, Cell and Science.
From only 159 registered patents in the US in 1989, Korea is now the third-highest patenting overseas country in the US, with 8,762 patents registered in 2009.
Along with multinational companies, there are about 250 domestic manufacturers, some involved in full-scale R&D.
Seoul is one of the world’s largest clinical research centres by trial numbers. The Korea bio hub centre has been established to increase networking among the around 20 bioclusters throughout the country, such as the High-tech Medical Complex (Daegu and Osong), Biomedical Complex (Songdo Incheon and Gyeonggi), Medical Industry Techno Valley (Wonju) and Daedeok Research Complex.
The Korea Institute for Industrial Economics and Trade expects the biotech industry to grow by over 20 per cent on average annually, considering the supply and demand of the last three years, but warns that investment levels in R&D are still low (reached $0.5bn in 2008, at just 1.2 per cent of the R&D investment of the US).
Trends and outlooks
BMI Healthcare regards South Korea as one of the most attractive pharmaceutical markets, both in the Asia Pacific region and globally, given its large size and the improvements to its regulatory regime required as part of the KORUS FTA.
Local pharma companies will suffer with the enforcement of intellectual property laws and will find it hard to compete in the new landscape. Many will not get government support, as they are not engaged in R&D and will see prices of generics plummet further. Litigation is likely to increase substantially, as domestic firms try to fight patents and price cuts. Competition from China and India will increase for manufacturers of generics, which will also have to invest in manufacturing capabilities to meet international standards. The alleged bias or protectionism towards domestic pharma will also reduce.
| Korea’s key players ($m) | |||
|---|---|---|---|
|
Ranking |
Company |
Sales |
R&D |
|
1 |
Donga Pharmaceutical |
684 |
43 |
|
2 |
Hanmi Pham |
539 |
59 |
|
3 |
Yuhan |
519 |
32 |
|
4 |
Green Cross |
476 |
31 |
|
5 |
Jeil Pharmaceutical |
328 |
9 |
|
6 |
Choongwae Pharma |
414 |
19 |
|
7 |
Chong Kun Dang |
271 |
24 |
|
8 |
Kwang Dong Pharmaceutical |
270 |
4 |
|
9 |
Handok Pharmaceuticals |
283 |
13 |
|
10 |
LG Life Sciences |
276 |
64 |
Source: Medi Pharms Today
| Top 10 pharmacos in Korea | |||
|---|---|---|---|
|
Ranking |
Company |
||
|
1 |
Pfizer |
||
|
2 |
Sanofi |
||
|
3 |
GlaxoSmithKline |
||
|
4 |
Novartis Korea |
||
|
5 |
Handok Pharmaceuticals |
||
|
6 |
MSD |
||
|
7 |
AstraZeneca Korea |
||
|
8 |
Janssen Korea |
||
|
9 |
Bayer |
||
|
10 |
Roche |
||
Source: Invest Korea (each company’s financial report [2008]); Daily Pharma News (April 7, 2008) Note: year-on-year basis
Korea’s pharma environment also remains uncertain for multinationals. The current policy of price cuts and, consequently, the disputes between companies and government over what is a fair price for an innovative drug, are set to continue.
Pharma expenditure has been high for years and in the past medicines were quickly approved into a huge reimbursable list with no cost-value considerations. So, the current delisting, including retroactive reassessments, is eating into profits.
The government’s vision for restructuring the pharma industry is based on two priorities: cost containment and ending illegal practices. Lengthy and litigious negotiations have resulted in launch delays for new drugs.
As in other markets, collaboration and licensing agreements are the way forward and the new regulatory status quo with FTAs with the US and EU can drive sales of branded products.
Biotech is one of the most promising sectors and with government investment and private funds returning to R&D, steady growth is expected..

The Author
Catarina Féria Walsh is a freelance journalist specialising in the pharmaceutical industry





