Market access is the process to ensure that all appropriate patients who would benefit, get rapid and maintained access to the brand, at the right price.
Market access is the process to ensure that all appropriate patients who would benefit, get rapid and maintained access to the brand, at the right price.
Success in practical terms means understanding fully the implications and requirements of each of the words in green in this definition.
Process
Ensure understanding of all processes that impact market access, both internal and external:
-
Payers have processes – at national level this includes HTA, pricing and reimbursement approval (including Value-based Pricing); and at local level, Drugs and Therapeutics committees (formulary inclusion decisions).
-
R&D process; a series of ‘decision gates’ to guide investment decisions throughout drug development to manage the huge cost of R&D (~$1bn) linked to the likelihood of success.
-
Companies may have a commercial launch excellence process; preparing the brand for the market, preparing the company for the brand, preparing the market for the brand.
Market Access needs good process, linking
-
Value identification, based on payer customer insights
-
Value creation through clinical and health economic outcomes and research (HEOR) data, and
-
Value communication through the Value Proposition & Value Dossier to the R&D and commercial ‘decision gates’; what gets done when (early enough for consideration in clinical study design) and how.
The market access process must link the requirements at global level, which guide the clinical development process, to the needs at local country level. The global value proposition and global value dossier (key deliverables from market access developed in collaboration with the key markets and across functions) must be adapted to specific market access customers at national and local level, as each healthcare system is unique.
Establish a measurement system to track progress and drive effective corrective action when necessary.
The key challenge is linking all these processes together – for payers, for R&D, for commercial and for market access, to deliver commercial success.
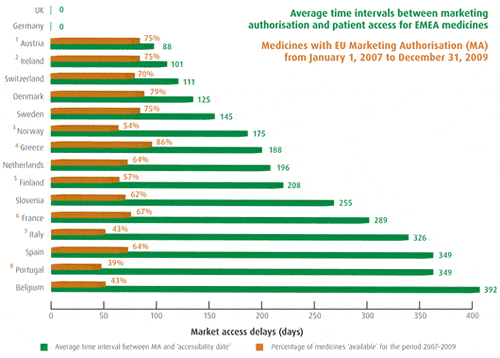
Patients W.A.I.T Indicator 2010 – Preliminary Report
Click on the image to enlarge
- Where Member Associations have collected data from their membership, the number of medicines considered are those for which companies provided information.
- Updated information is expected from France (latest update: October 2009); Greece, Ireland and Switzerland (latest update: March/August 2009); and Sweden (which communicated data that could not be accessed).
- For the purpose of the Patients W.A.I.T. Indicator, it is considered that Germany and UK allow access to medicines upon marketing authorisation – in these countries, no pricing/reimbursement process needs to be completed before new medicines can be prescribed to patients.
- 84 new medicines reported in the EU Medicines Register. For the following countries, the number of medicines considered are: 43 for Switzerland; 44 for Greece; 56 for Spain; 47 for Slovenia; 63 for France and 65 for Ireland.
Background/Clarifications:
- Austria: 63 new medicines were included in the Warenverzeichnis within an average 126 days after EU MA. As per April 2010: 37 medicines were included in the red box (within an average 85 days after EU MA), 17 were included in the yellow box (on average 296 days after MA); only three benefited from the green box conditions (on average 292 days after MA), and 29 were excluded from reimbursement (being included in the ‘no box’ after an average 152 days).
- Ireland: Medicines available in hospital only are accessible and not submitted to pricing/reimbursement procedures, and are therefore accessible upon marketing authorisation; excluding the 23 hospital only medicines, the average time needed to permit patient access is 184 days.
- Norway: For medicines that are not included in the positive list of reimbursed medicines, doctors can apply for reimbursement on an individual basis for patients that need the treatment. Some of the medicines listed as ‘individuals’ may also be in the process to get reimbursed (ie, ‘pending’), since medicines are reimbursed on individual basis during the time that reimbursement applications are being handled.
- Greece: Since 2006, new medicines are made available to Greek patients upon completion of pricing negotiations, and are then automatically eligible for reimbursement. This has significantly increased the availability rate and reduced delays in patient access.
- Finland: Calculation of average time intervals excludes two medicines, for which the time interval exceeds 2x the averages. With inclusion of these medicines, the average time interval would be 243 (instead of 208).
- France: Medicines with ATU status are available to (individual) patients before MA.
- Italy: Decisions on reimbursement are made at central level by AIFA. However, regions can make decisions relating to their budgets, which can indirectly lead to limiting ability of patients to access treatments (thus impacting on genuine patient access).
- Portugal: Until the introduction of prior approval for hospital-only medicines in 2006, no price/reimbursement approval process applied, since legislation imposes HTA evaluation prior to first acquisition of medicines by hospitals. It is expected that HTA evaluation will increase time needed to complete administrative processes, and delay patient access to new medicines.
Appropriate patients
Payers want predictability of patient outcomes and certainty of budget impact. So we need to be able to identify the patient population in numbers so you, and critically the payer, can count them. Patient population X price = budget impact.
The more certain we can be about the size of the patient population, the more confident payers will be in our prediction of budget impact.
One important aspect of identifying the appropriate patient is defining and identifying those patients most likely to be responders. Biomarkers are becoming increasingly important, in an attempt to try to limit the Numbers Needed to Treat (NNT), although this does not always hold true with some so-called personalised medicines having a higher NNT than non-biomarker medicines.
Medicines for orphan or ultra-orphan indications clearly have tiny patient populations, so while individual patient costs may be high, overall budget impact to the healthcare system is low.
Benefit
Benefit means improved health outcomes: outcomes that reflect the correct endpoints in eyes of payers. Historically, for pragmatic reasons, surrogate endpoints have been used. However, hard endpoints are increasingly required, with the US FDA stating that mortality may be a more appropriate endpoint than HbA1c control in diabetes, and overall survival preferred to progression free survival (PFS) in oncology.
In guiding clinical development to deliver payer value, we must focus primary and secondary endpoints on those specific outcomes that payers believe deliver value to the healthcare system and deliver true benefit.
Benefit must be expressed relative to Standard of Care (SoC), as perceived by the payer, not the company. Preferably, it would be expressed in real-life settings to show how the new medicine performs in more naturalistic environments, which reflect the value that will be delivered in real life.
Benefit also needs to be considered relative to ’emerging’ SoC – new interventions that are changing clinical practice that may not have been licensed when a Phase III trial was designed. This requires careful Phase III design to enable indirect treatment comparisons. In addition, network meta analyses must be conducted to answer these important questions.
SoC may vary between countries and regions. Phase III trials cannot cover every possible option for SoC and, therefore, at local level the market access plan must consider how the value proposition
and value dossier must be adapted to reflect local clinical practice, using modelling methods.
Benefit may also relate to improvements in quality of life (QoL). In this case it is vital to include effective measures such as EQ5D into Phase III clinical trials to collect utilities that can be reflected in cost per QALY analyses. If this hasn’t been done, all may not be lost. You may be able to map utilities from other disease-related QoL instruments to EQ5D.
Finally, you need to be clear about who benefits. Will all patients benefit or will some patient sub-groups benefit more than others? Payers will want to consider this in their assessment of benefit for the ‘appropriate’ patient.
Rapid access
We know that any delay in access has an impact on peak sales at the end of the life cycle. This can limit the profit available to reinvest in innovative new medicines, or to distribute to shareholders, which include many institutional pension and insurance funds.
There is a clear correlation between rapid access and commercial success.
So what do we mean by rapid access? Ideally we’re talking about access at Marketing Authorisation Approval to ensure that patients benefit quickly from innovative medicines. The reality is that in many countries there is a protracted delay while pricing and reimbursement approval takes place (see EFPIA chart). Even in countries with short delays for medicines, there can be a protracted and unwarranted delay for vaccines.
Maintained access
Increasingly payers are removing mature brands from reimbursement, where they believe cheaper alternatives are available, especially generics.
In addition, many payer processes involve review of earlier decisions, which means access can change during the life of a brand.
Market access planning and implementation is vital throughout the life cycle of the brand, not just at launch.
For many major brands, 85 per cent of their total value is derived from indications and forms not included in the very first marketing authorisation; for example, cancer medicines that are developed in multiple disease states (metastatic to adjuvant to neo-adjuvant) in multiple tumour types. Therefore access for new indications and new forms are vital elements in achieving the total life-time value of the brand vision.
Right price
The right price according to whom?
Medicines are priced on what the market will stand in terms of perceived value. Medicines have always been priced on value, not cost, because the major cost for any medicine is the $1bn+ in R&D costs to bring it to market, plus the need to amortise the cost of all
those medicines that fell by the wayside during development.
From the payers’ perspective, there is a need to consider ‘willingness to pay’, which can vary by disease area and perceived unmet medical need.
Increasingly, due to economic pressures, there is greater focus on ability to pay. There is an increasing need to justify that the benefit really is worth paying for, such as one month extra life in late-stage cancer treatment.
Price needs to reflect value. It is necessary to understand how healthcare systems value medicines and new developments. For example, the UK plans to introduce Value-based Pricing (VBP) for NCEs in 2014, based on a range of cost-effectiveness thresholds, which in turn are based on disease burden, level of innovation and wider societal benefit. But how will these be measured?
The response by NICE to the Kennedy report suggests it thinks it captures most of these criteria already.
Is the QALY the best way of measuring value for all situations? The reality is it may discriminate against those with late-stage illnesses and poor prognosis – patients with short life expectancy and poor quality of life. So in some cases the QALY may not be the right tool to use, as demonstrated by the Government instigation of the Cancer Drugs Fund in England.
How do you value new medicines at the beginning of their life, when the real value will be realised over their entire life cycle, and beyond when cheaper generics and biosimilars continue to deliver that value for more patients at a lower budget impact? The truth is that real price declines over time with inflation, price cuts, price/volume agreements, tendering and PAS.
We need ‘parametric’ VBP, variable over time. However, there is little trust in governments to give value-based price rises when they have a maximum four-year time horizon.
Market access is everyone’s responsibility throughout the life of the medicine from early phase R&D right through to loss of exclusivity.
 The Author
The Author
Colin Wight, Chief Executive at GalbraithWight.
He can be contacted on +44 (0)1323 482208 or at c.wight@galbraithwight.com