
The pharma sector is now well and truly the ‘biopharma industry’ with new data showing that biotech and emerging companies developing a majority of the drugs approved in 2018.
This is one of the key findings from a revealing new study of industry trends from IQVIA, The Changing Landscape of Research and Development, which examines drivers for change today and in the future.
The report found that so-called Emerging biopharma (EBP) companies patented almost two-thirds of the new drugs approved in and registered 47% of them, while large pharma companies patented just one-quarter of the total.
This is an enormous change in how new medicines are developed, and show that big pharma companies are now locked into depending on ‘external innovation’ to sustain themselves.
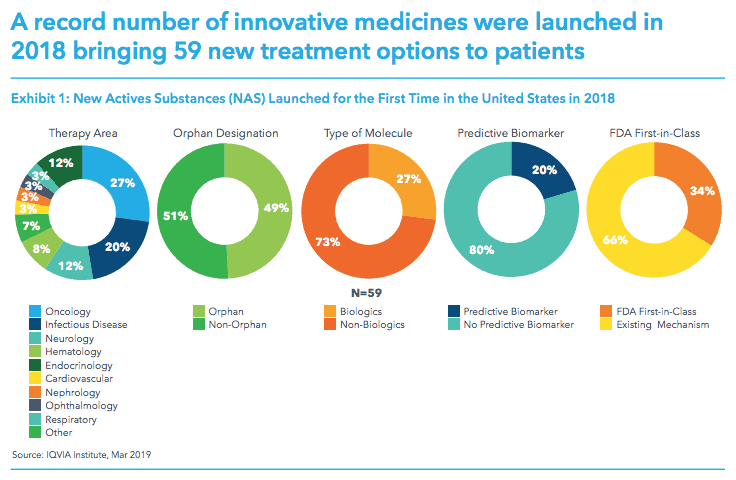
The report confirms that 2018 was a record-breaking year in terms of new medicines reaching the market with 59 novel treatments reaching patients in the US alone.
Among these drugs, 27% were treatments for cancer and its symptoms and 20% are for the treatment of infectious diseases.
Almost half had orphan drug status and over a third were identified by the FDA as first-in class therapies – such as Alnylam’s hereditary ATTR amyloidosis drug Onpattro (patisiran) the first RNA interference (RNAi) therapy and Lox/Bayer’s NTRK gene fusion – targetting Vitrakvi (larotrectinib).
However overall sector productivity – including the return on investment from its R&D spending – painted a worrying picture for the sector.
The report found that the composite success rate of clinical development from phase 1 trials to regulatory submission — based on the percentage of drugs successfully progressing to each next stage of development — fell to 11.4% in 2018, down from 14.4% in 2017.
This was below the average of 14% in the previous ten years, and all stages of clinical development saw declines in success rates in 2018, with phase 1 and phase 2 trial success both falling by 7−8%.
Success rates by development stage have all generally been consistent over the past decade, with 2015 an exceptional year, when the composite success rate exceeded 22%. While therapy classes and drug types under development have changed during the past decade, oncology has had slightly lower composite success rates (12%) than non-oncology (14.1%).
To examine the productivity of the clinical development process, IQVIA developed its own Clinical Development Productivity Index to measure trial success in relation to the effort invested in trials, and found rising failure rates in phase 1 the biggest problem.

Declining productivity in phase 1 was driven by declines in success rates of 7% and increases in trial complexity (which includes numbers of trial participants, eligibility criteria, research sites countries, and endpoints) of 6%. In 2018, complexity rose due to increases in all complexity elements excepting the number of trial sites and countries.
For instance, the number of patients expected to participate in clinical trials across the nine key therapy areas increased 10% over 2017 with growth influenced by an increase of the number of patients in phase 3 oncology and neurology trials.
The sector is working hard and investing billions in new approaches to cutting waste and failures in clinical trials, including investment in digital tracking technologies, but are yet to see any resulting improvement.
Over the next five years, IQVIA predicts that improvements in trial productivity will be driven by a handful of key trends such as biomarkers, pre-screened patient pools and predictive analytics.
Charts taken from the IQVIA report, The Changing Landscape of Research and Development.




