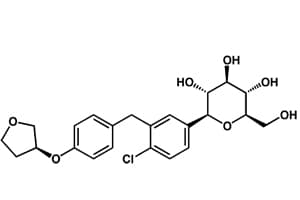
 The European Medicines Agency (EMA) has begun its review of Boehringer Ingelheim and Lilly’s new oral type 2 diabetes drug empagliflozin.
The European Medicines Agency (EMA) has begun its review of Boehringer Ingelheim and Lilly’s new oral type 2 diabetes drug empagliflozin.
The treatment is a sodium glucose co-transporter-2 (SGLT-2) inhibitor, the same new class of type 2 diabetes treatment as Bristol-Myers Squibb and AstraZeneca’s Foxiga and Johnson & Johnson’s canaglifozin.
SGLT-2 inhibitors work independently of insulin and appear to provide sustained improvements in blood glucose, whilst also alleviating the weight gain seen with other oral anti-diabetic agents (OADs).
“We are pleased the EMA has accepted our marketing authorisation application for a potential new treatment option to help patients better manage their type 2 diabetes mellitus,” said Prof Klaus Dugi, corporate senior vice president medicine, Boehringer Ingelheim.
The European filing follows empagliflozin’s recent submission to US regulators for approval, giving it a chance to catch up with its rival SGLT-2 inhibitors.
Bristol-Myers Squibb and AstraZeneca’s Forxiga (dapagliflozin) has already been approved in the EU, but delayed in the US, while Johnson & Johnson’s canagliflozin has been filed for approval in the US and Europe.
Empagliflozin is one of the four compounds covered by Boehringer and Lilly’s 2011 co-development agreement.
Early this year Lilly took back sole development and marketing rights to LY2605541 – a basal insulin product, but the partners are still working together on Tadjenta (linagliptin) and LY2963016, a second basal insulin analogue.




