
A vaccine to treat brain tumours was today designated the UK’s first ‘Promising Innovative Medicine’ (PIM) as part of a scheme to accelerate the availability of medicines for serious diseases.
Northwest Biotherapeutics received the designation for DCVax-L, a personalised immunotherapy that targets malignant gliomas, including glioblastomas – the most severe form of the cancer.
PIM status was introduced in the UK in March this year as part of the Early Access to Medicines scheme. It allows companies to have innovative treatments used in an NHS population once the Medicines and Healthcare Products Regulatory Agency (MHRA) has determined the benefits outweigh the risks.
This bypasses the usual route for new drugs for NHS use, which includes approval from the European Commission and then a cost effectiveness review by the National Institute for Health and Care Excellence (NICE) or the Scottish Medicines Consortium (SMC).
UK Life Sciences Minister George Freeman said: “The designation of the PIM is the first, crucial step in developing cutting-edge medicines sooner, giving real hope to patients and their families.”
PIM has been likened to the US FDA’s successful ‘breakthrough’ class and is reserved for new medicines to treat serious life-threatening conditions that are likely to offer a major advantage over currently available treatments.
DCVax-L – a therapy manufactured from a patient’s dendritic cells pulsed with tumour cell lysate – qualifies due to the serious nature of malignant gliomas, while the drug has also demonstrated its effectiveness in early clinical trials.
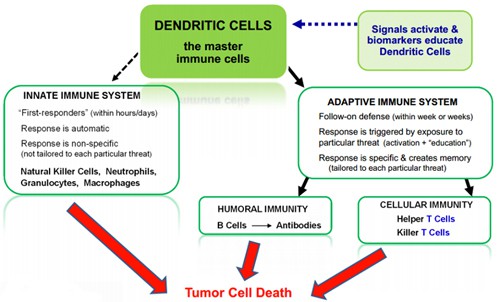
The process of Dendritic Cell immunotherapy (Northwest Biotherapeutics)
The first designation will come as a relief to the UK Government, which was criticised by some areas of the industry for making the scheme funded by companies who produce the medicine rather than through national reimbursement.
At the time the scheme was announced the BioIndustry Association’s CEO Steve Bates lauded its intent, but said it ran the risk of being “under-utilised” due to the nature of its funding.
The cost hasn’t halted Northwest Biotherapeutics’ ambitions, however, and the company is set to benefit from the experience of having a drug used in a real-world NHS setting, potentially accelerating the process to gain a full licence complete with national reimbursement.
As for next steps, the Department of Health confirmed Northwest Biotherapeutics will continue to develop the medicine and, if proven in trials, it will be eligible to apply for step two of the UK Early Access to Medicines Scheme. It will then go through formal medicines licensing.




