A new survey of small-to-medium sized (SME) pharma companies shows dwindling confidence in the UK government’s handling of Brexit negotiations, and growing fears for their businesses and patients.
The Ethical Medicines Industry Group (EMIG) represents SME pharma in the UK, and conducted its second ‘Brexit Barometer’ in June, shortly after a transition deal was agreed between the UK and EU27 in March.
Despite this deal, which gives the UK two more years on virtually the same trade and regulatory terms as now, there are signs of greater pessimism among respondents compared to the first survey conducted in the spring.
In that survey, 37% of the 70 respondents said they believed the UK would reach a deal with the EU that would result in lower access to EU market, but with more flexibility to negotiate trade deals with the rest of the world, a so-called ‘Canada’ option.
The new survey of 50 respondents shows sentiment has shifted, with a sharp increase in those saying “We do not have any expectation,” up to 26% from 18% last time.

This likely reflects growing confusion and frustration at the lack of clarity over the UK government’s own position as the Brexit date of 29 March 2019 looms ever closer.
It could also reflect fears that ‘Brexiteers’ have the upper hand in many of the internal battles within the Conservative party. They are pushing for a ‘No Deal’ option if the EU27 doesn’t offer what they consider a satisfactory deal, which includes a ‘frictionless border’ but with the UK also leaving the single market and customs union.
EMIG’s chairman Leslie Galloway (pictured) agreed that the survey reflected frustration with the negotiations among member companies.

“The whole thing is a mess, that is the one consistent thing people say about the Brexit negotiations. This second survey was conducted shortly after the transition deal was agreed, so I had expected more optimism, but that’s clearly not the case.”
The survey comes shortly after a number of large multinational companies, including Airbus and BMW warned they would be forced to shift investment out of the UK if a workable trade and customs deal could not be agreed.
Prime Minister Theresa May is in Brussels today to brief her EU counterparts on the UK’s position, the last time before October, when both sides hope to reach a final agreement on post-Brexit relations.
May has secured the UK Parliament’s backing for Brexit legislation, however there is still a gulf between the UK’s demands on future relations and the EU27’s position, with several member states expected to block demands for frictionless trade with a UK outside the single market and customs union.
May is expected to tell other European leaders that she will have a clear agreement within her own government by the end of July, and be in position to publish a White Paper setting out its goals on areas such as trade, customs, agriculture, and medicines post-Brexit.
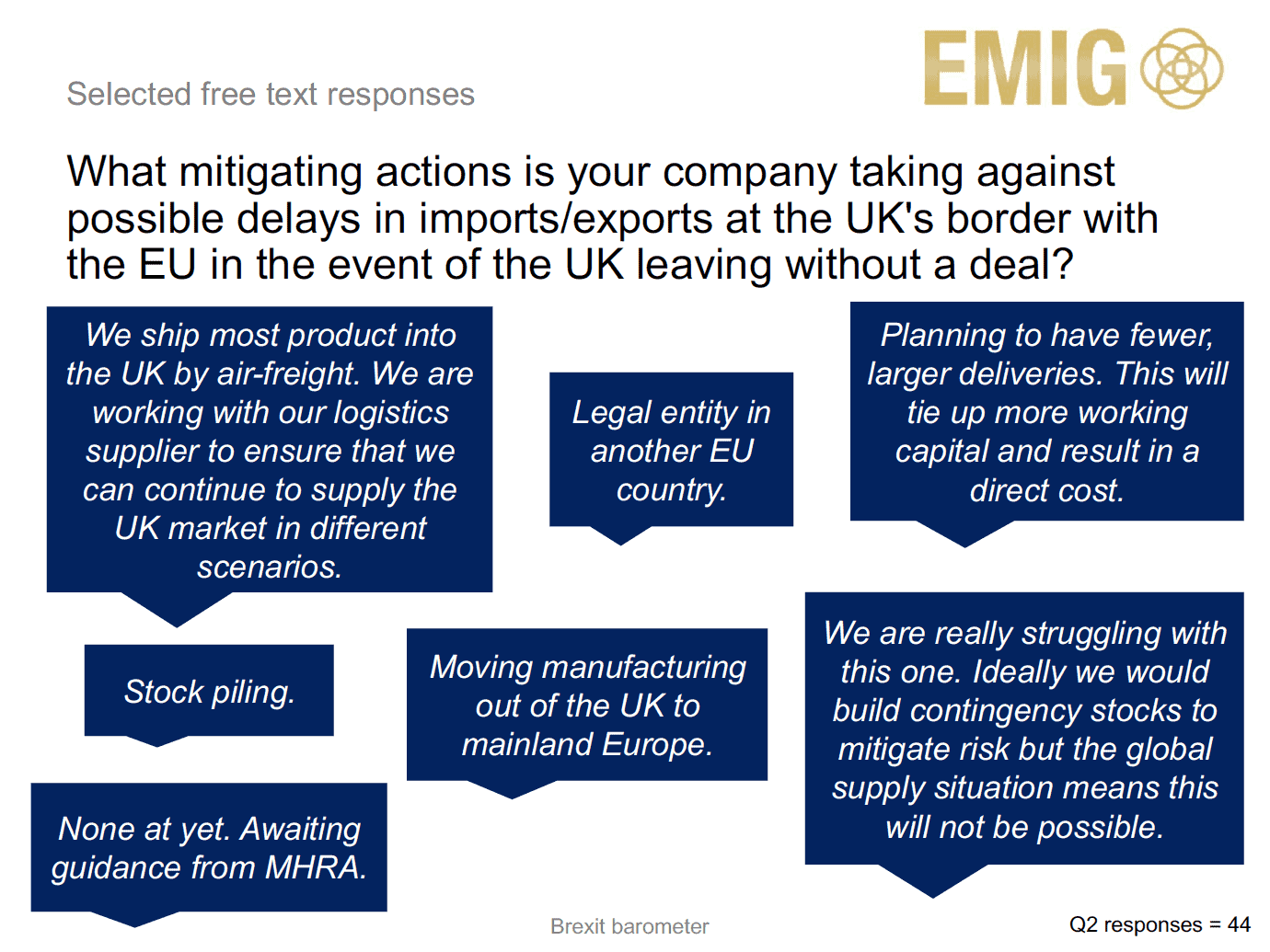
In the meantime, big and small pharma companies operating in the UK are having to make plans for all possible scenarios.
Leslie Galloway says some smaller companies feel forced to gamble on a deal being struck at the last minute, as they are unable to spend time and money on planning for a ‘No Deal’ scenario on the same scale as big pharma companies like GSK and AstraZeneca.
The latest Brexit Barometer shows most respondents say they have spent no more than £50,000 on Brexit preparations so far, although a small proportion have spent far more.
“I was asked by one company for advice on the best course of action – but no-one is able to clear view of how things will work out. The only thing companies can do is to follow what the EMA says, which is to assume a hard Brexit, in which the UK is a ‘third country’,” adds Galloway.
On the extensive ‘to do’ list for the industry is moving marketing authorisations out of the UK and establishing new batch release testing facilities, and making non-UK manufacturing arrangements. The survey shows there are a variety of tactics companies are taking to mitigate the potential impact of Brexit, but Galloway says there is “no magic formula” in such an uncertain environment.
These extra costs for SMEs coincide with the new EU Falsified Medicines Directive, a new safety and medicines tracking system which comes into force in February 2019.
Companies, regardless of their turnover, must pay fees every year, with those with 12 or more product licences paying €20,000, or more than double this amount if they sign up later than June 2018.




