
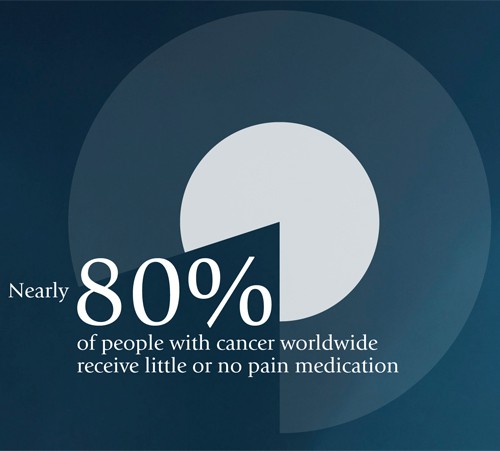
Cancer pain can be well controlled in almost all cases, but nearly 80% of people with cancer worldwide receive little or no pain medication.
It is estimated that up to half of all cancer patients experience some degree of cancer pain and a significant proportion experience pain significant enough to disrupt sleep, mood and daily activities. Ongoing or persistent pain is associated with anxiety, depression and reduced quality of life.
Cancer pain, which has many potential causes, is often caused by the tumour itself invading or compressing organs or tissues which can cause anything from mild to extreme pain. Some tumours are also known to release factors that make nociceptors (pain receptors) hypersensitive or responsive to normally non-painful stimuli. Invasion of bone tissue is the most frequent cause of cancer pain and can be very debilitating, while bone metastases can also increase the risk of bone fractures. Tumours can directly or indirectly compress or inflame parts of the central or peripheral nervous systems which can cause mild to severe pain known as neuropathic pain. Tumours may also compress blood vessels causing pain, oedema and potentially thrombosis. Up to 15% of deep vein thromboses are caused by tumours and can often be the first sign that a malignancy is present. Tumours may also invade or compress organs (visceral pain) or structures such as ligaments, muscles and tendons.
Concurrent medications could see doses, and the inevitable side effects, of classic opioids reduced
A significant proportion of cancer pain however is caused by the interventions and procedures – such as chemotherapy, hormonal therapy, radiotherapy and immunotherapy – used to diagnose and treat the cancer itself and patients may also experience post-surgical pain or pain from diagnostic procedures such as lumbar punctures and biopsies.
First paracetamol and NSAIDS
WHO guidelines say oral administration of non-opioid analgesics such as paracetamol and NSAIDs should commence upon first sign of cancer pain. If these fail to control the condition or disease progression worsens the symptoms, treatment should be escalated to include mild opioids such as tramadol or codeine. If the pain is still not controlled then strong opioids such as morphine should replace the milder ones. If the initial pain is severe then strong opioids in combination with non-opioid analgesics are recommended straightaway.
In fact over two-thirds of the drugs listed as registered or marketed globally for cancer pain on the Adis R&D Insight database are opioid agonists. In addition to the traditional opioids such as morphine and codeine, many of these are proprietary formulations of fentanyl, another powerful opioid. These include buccal, intranasal, transdermal, sublingual and transmucosal formulations of the drug. Nine of the 15 drugs listed as being in phase III development are also opioid agonists but some distinctly different mechanisms of action are also being developed here.
Phase III: opioid agonists
GW Pharmaceuticals has developed a buccal spray known as nabiximols or Sativex, which comprises delta-9-tetrahydrocannabinol (27mg/mL) and cannabidiol (25mg/mL), as an analgesic agent for the treatment of neuropathic pain, spasticity, cancer pain and for neuroprotection in Huntington’s disease. The product, which contains soft extracts from Cannabis sativa, is primarily indicated for the treatment of multiple sclerosis (MS)-related spasticity. In Canada, the product is also launched for the adjunctive treatment of cancer pain and neuropathic pain in adults with MS. Clinical trials are underway in cancer pain and neuropathic pain in the US and Europe.
WEX Pharmaceuticals (formerly International Wex Technologies Inc.) is developing tetrodotoxin, a sodium channel blocker, for several different indications. Tetrodotoxin is a complex non-protein toxin extracted from the blowfish known as fugu in Japan. The non-opiate nature and numbing effect of tetrodotoxin may provide advantages over currently used morphine or codeine in patients with constant pain symptoms. An intramuscular formulation is being developed for the treatment of cancer pain (as Tectin) and is in clinical trials in Canada.
Tetrodotoxin is also being investigated for the treatment of neuropathic pain and as an anaesthetic. It appears the company is in the process of optimising a formulation of the agent for further development. WEX Pharmaceuticals is conducting a phase I trial comparing liquid and lyophilised subcutaneous formulations of the agent. Four-day treatment with intramuscular tetrodotoxin was reported to be safe and well tolerated at doses up to and including 30µg twice daily in patients taking opioids and other medications for severe cancer pain. In this study, 98% of adverse events were classified as ‘mild or moderate’ and did not raise specific safety concerns.
Grünenthal GmbH is developing cebranopadol, a centrally active, small molecule analgesic for the oral treatment of pain. This first-in-class compound shows agonist activity on the nociceptin receptor. The compound is being developed in liquid, capsule and tablet formulations. In the EU, cebranopadol is in phase III development for cancer pain, and phase II development for the treatment of musculoskeletal pain, back pain and diabetic neuropathies. A follow-on compound of cebranopadol, called GRT 6006, has also entered clinical development. Grünenthal is conducting a randomised, double-blind phase III trial to compare the analgesic efficacy and tolerability of cebranopadol and prolonged-release morphine for the treatment of patients with chronic, moderate-to-severe cancer pain.
Phase II: novel mechanisms investigated
Following the same trend, two-thirds of the phase II pipeline is also comprised of opioids. Again however, we see some novel mechanisms being investigated such as alpha-2 adrenergic receptor agonists and serotonin-1A receptor agonists.
Dexmedetomidine, an IV infusible dextroisomer of medetomidine, is a beta-2 adrenergic receptor agonist that has been launched in the US, Canada, Japan, South Korea, Australia and some countries in the EU as a sedative for intensive care patients. Sublingual and transdermal formulations of dexmedetomidine are undergoing clinical development with Recro Pharma for the adjunctive management of pain. Recro is also conducting phase II development of an intranasal formulation for the treatment of cancer pain and post-operative pain. In clinical trials, dexmedetomidine was well tolerated.
Pierre Fabre is developing befiradol, a selective serotonin-1A receptor agonist, for the treatment of chronic pain. This compound produced powerful central analgesia in animal models of pain, through the neuroadaptive mechanisms of inverse tolerance and cooperation with nociceptive stimulation. Befiradol is thought to have analgesic effects comparable to those of high-dose opioid painkillers, but with fewer and less prominent side effects. Oral befiradol is in phase II development in Europe for the treatment of cancer pain and neuropathic pain.
Focus on delivery systems
Adis R&D Insight only lists 3 compounds in phase I development for cancer pain, two opioids and a cannabinoid receptor agonist. NEOMED is developing an orally available cannabinoid 1 and 2 (CB1/CB2) receptor agonist known as NEO 1940 for supportive care therapy in patients with cancer. NEO 1940 is being developed to treat cachexia, cancer pain, nausea and vomiting. Phase I development is underway and a phase II trial in cancer patients in Canada is planned.
While opioids look set to dominate this treatment landscape for many years to come, much work is being done to improve the delivery systems of these drugs. Additional research is focusing on concurrent medications which could see the doses of classic opioids, and the subsequent inevitable side effects, reduced, which would greatly benefit this already suffering patient population.





