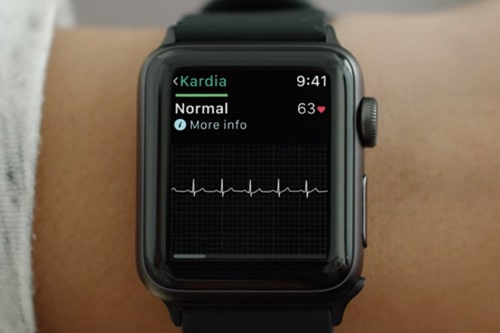
AliveCor has become the first company to secure US approval for a wearable medical device that works alongside the Apple Watch.
The FDA gave a green light to the company’s Kardia Band, a real-time electrocardiogram (ECG) housed in a watch strap – which retails for £199 in the UK ($199 in the US) – that pairs with its Kardia Watch app on the Apple Watch.
AliveCor already sells an FDA-approved £99 device called KardiaMobile, a strip which attached to the back of a smartphone or in a separate carry pod and requires the user to place two fingers of each hand on the strip for a 30-second reading.
The new device is more discreet and extends the technology into continuous rather than on-demand monitoring “in order to quickly detect normal sinus heart rhythms and atrial fibrillation (AF), the most common heart arrhythmia” according to the company, which is led by former Microsoft and Google exec Vic Gundotra.
Kardia Band has two main functions. In one, the user starts the app and – after making sure they are sitting in a relaxed fashion – presses a button on the wristband to start a 30-second ECG recording, with feedback on whether the heart rhythm is normal.
Secondly – thanks to new software called SmartRhythm – the device also monitors heart rate continuously, and can prompt the user to carry out a 30-second monitoring when abnormal heart rhythms are detected. The software “uses artificial intelligence in concert with inputs from Apple Watch’s heart rate and activity sensors to continuously evaluate the correlation between heart activity and physical activity,” says AliveCor.
Kardia Band can also capture heart activity data and relay it to the user’s doctor to help with diagnosis and treatment plans, and voice memos can be taken to keep a record of other information such as palpitations, shortness of breath, dietary habits and exercise patterns.
“This is a paradigm shift for cardiac care as well as an important advance in healthcare,” according to Ronald Karlsberg, a cardiologist at Cedars Sinai Heart Institute and David Geffen School of Medicine UCLA who sits on AliveCor’s medical advisory board.
“Today, EKGs are available only in offices and hospitals, using complex equipment, and usually only after a life-threatening event, for example a stroke. With an EKG device on the wrist, AF can be detected wherever the patient is, 24 hours a day.”
In September, AliveCor presented data from studies at the European Society of Cardiology Congress In Barcelona showing that patients using Kardia Mobile were four times more likely to have their AF detected, and that the diagnostic accuracy of the device rivalled that of physicians.




