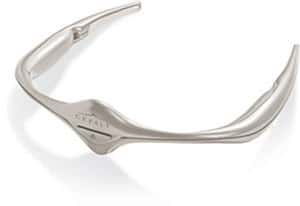
The US FDA has approved a drug-free regimen to treat migraines that involves the use of an electronic headband.
Known as Cefaly the battery-powered device is worn across the forehead and behind the ears and applies an electric current to the skin to stimulate the trigeminal nerve, which is associated with migraine headaches.
The approval covers use of Cefaly in patients aged 18 and over and the device should only be used once per day for 20 minutes.
Christy Foreman, part of the FDA’s Center for Devices and Radiological Health, said Cefaly offers an alternative to medication in the treatment of migraine, which involves an intense throbbing pain the head and is thought to affect 10 per cent of the world’s population.
“This may help patients who cannot tolerate current migraine medications for preventing migraines or treating attacks,” added Foreman.
Manufactured by Belgian medical device company STX-Med, Cefaly has demonstrated its effectiveness in a clinical study involving 67 individuals who experienced more than two migraine headache attacks a month and who had not taken any medicines to prevent migraines for three months prior to using the device.
This study showed that people using Cefaly experience fewer migraines per month and used less medication than those on placebo.
These findings were corroborated by a patient satisfactions study, which showed that just over half of 2,313 users were willing to buy the device for continues use.
The approval provides competition to current drugs available to tackle the symptoms of migraine, including injectable therapies, nasal sprays and oral drugs, although use of the latter two can be limited due to the nausea associated with migraine while the former can be invasive and difficult to administer.
This has meant focus has moved to device-type treatments, including Cefaly and sumatriptan skin patch Zecuity, marketed by pain specialist NuPathe, which is now at the centre of an attempted takeover by Teva.




