Eli Lilly has been granted approval in the EU for Amyvid, a solution to diagnose Alzheimer’s disease.
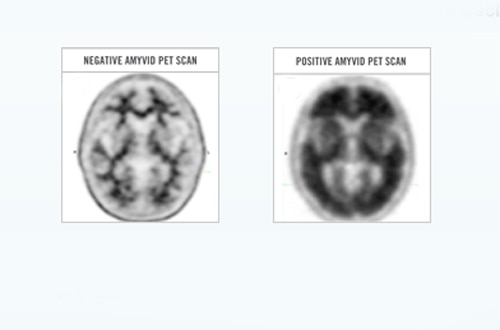
Amyvid (florbetapir [18F]) is used as an imaging agent during positron emission tomography (PET) scanning to detect the presence or absence of beta-amyloid plaques in the brains of adult patients with cognitive impairment.
At the moment, doctors rely only on a clinical evaluation to decide whether patients with cognitive impairment are suffering from Alzheimer’s disease or some other cause of cognitive decline, so being able to see whether amyloid plaques are present in the brain will help the accuracy of diagnosis.
Plaques are a hallmark of Alzheimer’s disease, although they can also occur in other neurological conditions and as a consequence of the normal ageing process, so Lilly stresses that Amyvid should only assist a clinical diagnosis and not be the sole criterion.
Prior to Amyvid becoming available it was only possible to detect amyloid in the brain after the patient died at autopsy.
“Amyvid is the first and only diagnostic tool approved for use in the European Union that can show the presence or absence of beta-amyloid neuritic plaque density in the brain,” commented Diane Bakaysa, who heads development of the Amyvid brand at Lilly.
“This is important because it is estimated that one in five patients clinically diagnosed with Alzheimer’s disease during life were ultimately misdiagnosed and do not exhibit Alzheimer’s disease pathology upon autopsy,” she added.
Lilly secured approval to market Amyvid in the US last April at its second attempt, after a first filing was rejected on concerns that the scans achieved with the imaging agent could be hard to interpret correctly without adequate technician training.
Lilly allayed those concerns by developing, with assistance from the FDA and nuclear medicines experts, a training programme that must be taken before Amyvid scans are interpreted.
Analysts have suggested Amyvid’s sales potential is limited, despite the rising incidence of Alzheimer’s disease around the world, because it cannot provide a definitive diagnosis and is not yet eligible for reimbursement by some health systems, including the Medicare and Medicaid programmes in the US.
A reimbursement review process at the Centers for Medicare & Medicaid Services (CMS) started in October but is expected to take some time to conclude, as it would involve overturning a decade-long rule that limited PET imaging reimbursement in the US.
The CMS may change its position given that competing PET imaging agents for Alzheimer’s are coming through development, notably GE Healthcare’s flutemetamol and Bayer’s florbetaben, both of which are in phase III.
Sales are expected to be modest – perhaps around $100m a year while reimbursement is absent – although Lilly is making use of Amyvid in its clinical trials programme for Alzheimer’s therapeutics.
These include anti-amyloid antibody solanezumab, which failed two phase III studies last year but remains in development in earlier-stage Alzheimer’s patients, and a BACE inhibitor which is in phase II.




