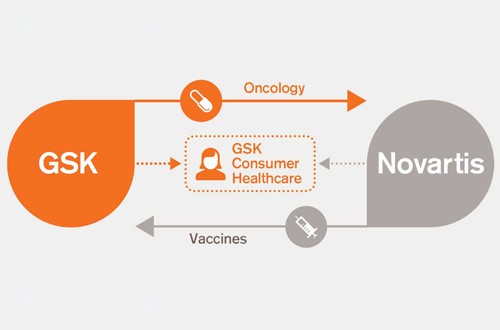
GlaxoSmithKline (GSK) and Novartis have completed the swap of vaccine and cancer drug businesses and the creation of a joint consumer healthcare unit after Novartis agreed to divest its BRAF and MEK inhibitor drugs.
Under the terms of the deal GSK is paying $5.25bn in case for Novartis’ vaccines business while Novartis is stumping up $16bn for GSK’s oncology business.
The two companies are also pooling their resources in consumer health, creating a joint venture that will be 63.5% owned by GSK. Details of the asset swap were first revealed last April.
Novartis claims ownership of several marketed products – including kidney cancer therapy Votrient (pazopanib) and Tyverb (lapatinib) for breast cancer – and also has opt-in rights to GSK’s current and future oncology R&D pipeline. GSK walks off with Novartis’ non-influenza vaccines business including meningitis B shot Bexsero.
GSK said it will walk away with $7.8bn in proceeds from the transaction and will return £4bn (just over $6bn) to its shareholders – who saw the value of their holdings plummet over the course of 2014 on the back of the Chinese bribery scandal and pressure on GSK’s flagship respiratory business in the US.
It also said that up to $1.5bn of the oncology unit’s purchase price is tied to the COMBI-d trial of BRAF inhibitor Tafinlar (dabrafenib) and MEK inhibitor Mekinist (trametinib), which generated positive overall survival data and was reported earlier this month.
Tafinlar and Mekinist have already been given approval in the US on the condition that GSK carried out COMBI-d to prove the efficacy of the combination, and the $1.5bn reserve is likely linked to the FDA’s review of that data.
As a condition of the oncology unit deal, Novartis has agreed to divest all its own BRAF and MEK inhibitors – LGX818 and MEK162 respectively – to Array BioPharma to avoid creating a monopoly in this area. Novartis must also provide transitional support to Array to ensure that the drugs have a good chance of making it through development, according to the agreement.
GSK and Novartis were two of just three groups working on RAF/MEK combinations to treat melanoma, with a dual therapy based on Roche’s BRAF inhibitor Zelboraf (vemurafenib) and Exelixis’ MEK inhibitor cobimetinib under regulatory review and due to for approval by the FDA in August.
The completion of the transaction “represents a major step forward in the group’s strategy to create a stronger and more balanced set of businesses across pharmaceuticals, consumer healthcare and vaccines,” said GSK chief executive Sir Andrew Witty.
GSK said it intends to report its first quarter results and hold an investor meeting on 6 May, at which it will provide 2015 earnings guidance – which it held off from during its 2014 results meeting last month – and profile the restructured group.




