Rare diseases, such as cystic fibrosis, are as economically viable for pharma as more common conditions, according to a new report.
The Economic Power of Orphan Drugs from Thomson Reuters claims that, when comparing the total value of orphan drugs from 1990 to 2030, they have the potential to generate as much lifetime revenue as mainstream drugs, thanks to government incentives, shorter clinical trials and high rates of regulatory success.
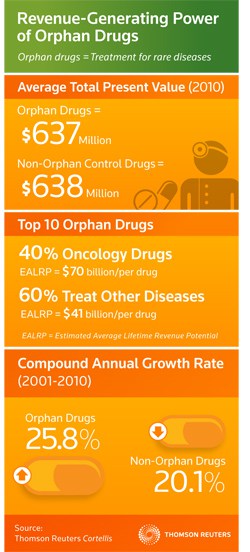
An increased focus on personalised, precision treatments has also increased attention towards orphan drugs, with the market now worth $637m compared to the $638m market for a matched control group of non-orphan drugs, Thomson Reuters said.
 The near parity comes despite orphan drugs having a much smaller market for each medicine. The European Medicines Agency (EMA) awards orphan drug designation for medicines that treat conditions affecting no more than 5 in 10,000 people, while in the US the FDA requires fewer than 200,000 people to be affected by the condition.
The near parity comes despite orphan drugs having a much smaller market for each medicine. The European Medicines Agency (EMA) awards orphan drug designation for medicines that treat conditions affecting no more than 5 in 10,000 people, while in the US the FDA requires fewer than 200,000 people to be affected by the condition.
In total, there are thought to be about 7,000 rare diseases worldwide affecting a combined US patient population of about 25m people.
In contrast, conditions like asthma and diabetes are estimated to each affect about 25m people in the US.
“This new data gives economic validity to the importance of targeting rare diseases in the global pharmaceutical market,” said Dr Kiran Meekings, life sciences consultant at Thomson Reuters and co-author of the report.
Among the orphan drugs highlighted in the report for achieving financial success is Roche/Genentech’s MabThera (rituximab), marketed as Rituxan in the US.
The drug, which is indicated to treat such rare conditions as Wegener’s granulomatosis (WG) and microscopic polyangiitis (MPA), is “expected to garner more than $150bn in revenue over its lifetime, the majority of which is for orphan indications”, according to Thomason Reuters.
Elsewhere, the report cites the industry’s most expensive drug, Alexion’s Soliris (eculizumab), which costs more than $409,000 per year for treatment of paroxysymal nocturnal haemoglobinuria (pNH), a rare life-threatening blood disease. Last year its sales stood at $541m, suggesting high prices can offset the limited patent population.
The market for orphan drugs is an expanding one too, with an estimated 250 new rare diseases identified every year, growth that has seen orphan drug revenues climb 25.8 per cent between 2001 and 2010, while the market for non-orphan drugs grew only 20.1 per cent.
“We expect the orphan disease business model to sustain a competitive edge over the traditional primary care business model in the future,” said Brian Lester, senior analyst and managing director of the life sciences group at the financial services firm Manning & Napier.




