Payments from a branded drug manufacturer to a generic maker to delay market entry can lead to broadly inflated prices across a therapeutic category, according to new research.
The findings suggest that efforts to investigate and ban pay-for-delay deals are justified, says study author UK health economist Dr Farosat Bokhari from the University of East Anglia.
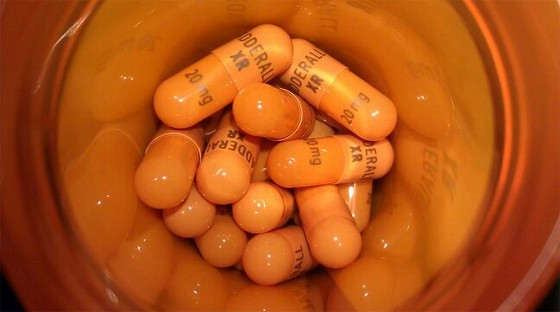
The study models a deal that delayed generic competition to Shire’s attention-deficit hyperactivity disorder (ADHD) drug Adderall XR in the US and concluded that percentage price increases are at least four-fold higher when a generic is not available in the market, compared to when the generic is available but the branded and generic firms jointly set their profit-maximising price.
Moreover, the price inflation affects other drugs in the ADHD category, so there is a cumulative impact on the amount of money being spent on drugs to treat the condition, according to the model.
In the absence of Adderall XR, the price of rival ADHD brand Concerta (methylphenidate) from Johnson & Johnson would be around 5 per cent higher – at $101 per month instead of $96.45 – while the prices of most other ADHD drugs would be higher with an average increase of almost 4.6 per cent.
“Pay-to-delay is a problem in the immediate future for health services in the US and Europe, and in the long run for taxpayers,” says Dr Bokhari, who believes the situation exposes the current tension between patent and antitrust law.
“While the monthly price increases may not seem huge, when you take into account the number of people using these treatments even modest increases have a significant impact on consumer welfare and add millions a year to their overall cost,” he adds.
The model focuses on the period between the start of patent litigation between Shire and Barr (now part of Teva) and Impax in 2002/2003 and the launch of multiple generics in October 2009 when a six-month exclusivity period granted by Shire to Barr came to an end.
Pay-for-delay deals are also on the rise, says Bokhari. For instance, there were three agreements involving a restriction on generic entry and a payment to the generic maker in 2005, but the tally rose to 19 in 2009, 31 in 2010 and 40 last year, according to the US Federal Trade Commission (FTC) which estimates the deals cost US consumers $3.5bn a year.
Antitrust regulators are placing the agreements under increasing scrutiny, and earlier this month it emerged that nine pharma companies are facing fines in the EU. Of these companies, Lundbeck is facing a penalty of €240m.
Meanwhile, the UK Office of Fair Trading has accused GlaxoSmithKline of blocking the entry of generic versions of its Seroxat (paroxetine) antidepressant by paying generic drugmakers not to launch, and the FTC is suing several pharmaceutical firms in a series of cases, one of which is due for a verdict by the US Supreme Court later this month.
The US Congress also has multiple bills in play which are seeking to render pay-for-delay deals illegal, although as yet none have passed into law.
“Drug companies argue that … these agreements benefit consumers by enabling generic versions to come onto the market sooner than they would normally have, for example if licensed entry has been allowed at a later date but before the patent expires,” comments Dr Bokhari.
“But while the deals may be beneficial to some extent, in that they might save courts and administrative bodies, such as patent offices, time and effort, they allow branded drug firms to charge monopoly prices and in a typical deal there may be a two to three year delay in a cheaper version becoming available.”





