
England’s healthcare cost effectiveness watchdog NICE has returned to its proposal to charge pharma companies for appraisals of drugs – but says smaller companies will have a 25% fee discount.
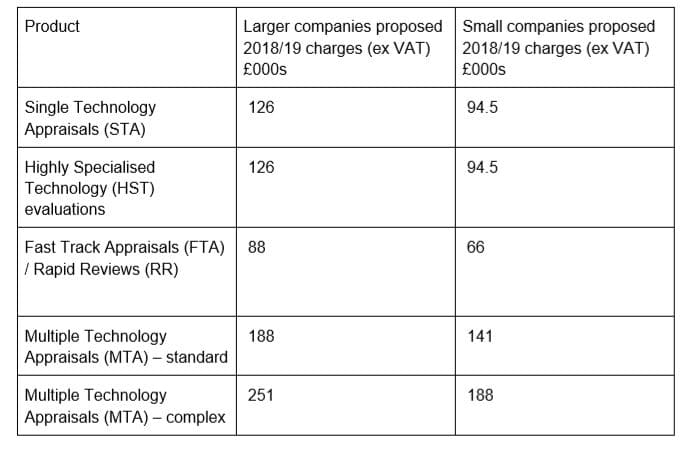
NICE is proposing that bigger companies pay £126,000 ($160,000) for a single technology appraisal or a highly specialised technology appraisal (for rare disease drugs), or £94,500 for smaller companies.
NICE first proposed the idea of introducing fees in 2016, but met opposition from industry on a number of fronts – including calls for reforms of its methodology in exchange for pharma accepting the charges.
Another complaint raised at the time was that the fees would hit smaller companies hard – and possibly even wipe out profits from launching in the UK, and these issues saw the plans shelved for some time.
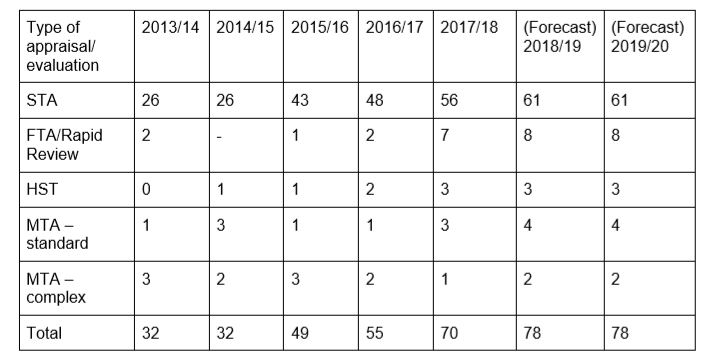
However NICE needs to raise funds from industry, as it has an ever growing workload and a shrinking UK government budget allocation. In 2013/14, it received £66.4m in government funding, but its budget has been progressively cut back by more than 20% to £51.2m in 2018/19.
NICE’s table showing its growing workload, with the number of single technology appraisals having already more than doubled since 2013/14
NICE’s table showing the proposed charges to industry
These proposals have now been put out to consultation until Friday 14 September, and would come into force on 1 April 2019, the start of the NHS financial year.
NICE and the Department of Health and Social Care (DHSC) are making it clear that the charges are for ‘cost recovery’ and don’t want them to be seen as a revenue stream as such.
NICE says it expects to generate around £10.5m annually, with the 25% discount for small companies taking a relatively small £0.3m slice out of that total.
Also contained in the consultation is a proposal for this new income to fund more appraisals – something which NICE believes is a silver lining to the charges.
The consultation states:
“DHSC is considering the potential for broadening the scope of referrals to NICE which, if agreed between DHSC, NHS England, NICE and industry, could result in up to 20 additional technology appraisals per year.”
Industry association the ABPI hasn’t yet responded to the proposals but greater capacity in NICE would be welcome – perhaps especially in the highly specialised technology category, where there are more and more products reaching the market, and limited NICE capacity to review them.
Industry objections and calls for reform of NICE – in particular its QALY-based methodology – forced the cost effectiveness watchdog to shelve the fees idea back in 2016. This consultation also includes plans to allow NICE to draw experts for its appeal panel from across the UK, and not just England, but no further changes. However even if there are protests from the industry, the continuing cuts to NICE’s budgets means this time the changes are likely to go ahead.
Read the consultation in full here:




