
The UK government has been highlighting the global AMR challenge since 2014 Source: Public Health England
The UK is to try out a subscription-style payment model aimed at incentivising pharma and biotech companies to develop new drugs needed to tackle resistant infections.
Resistant bacteria already cause more than 700,000 deaths globally every year, a trend exacerbated by misuse of antibiotics in some regions.
The UK government has taken a lead among nations in addressing the growing dangers of ‘superbugs’ and antimicrobial resistance (AMR) with the need for new classes of antimicrobials one of the main action points.
However, as health systems aim to reserve new antibiotic treatments for use as ‘last resort’ when existing drugs have failed, there is little financial incentive for commercial investment in the field.
This has led many public health leaders to talk about ‘market failure’ in antibiotic R&D, while the industry prefers to term it a ‘broken model’, not wanting to take blame for the drop off in new therapies in recent decades.
Pharma and biotech have been wary of some new suggested models to incentivising antimicrobial R&D, including one unpopular suggestion of a levy on pharma companies to raise money for a global prize fund for new antimicrobial treatments.
The new subscription trial is much more welcome in the industry, and will be led by NICE and NHS England and Improvement. This model see pharmaceutical companies paid upfront for access to drugs based on their usefulness to the NHS.
This will make it more attractive for companies to invest the estimated £1bn ($1.25bn) needed to develop a new drug, as they will still be paid for the drug even though it may rarely be used in patients.
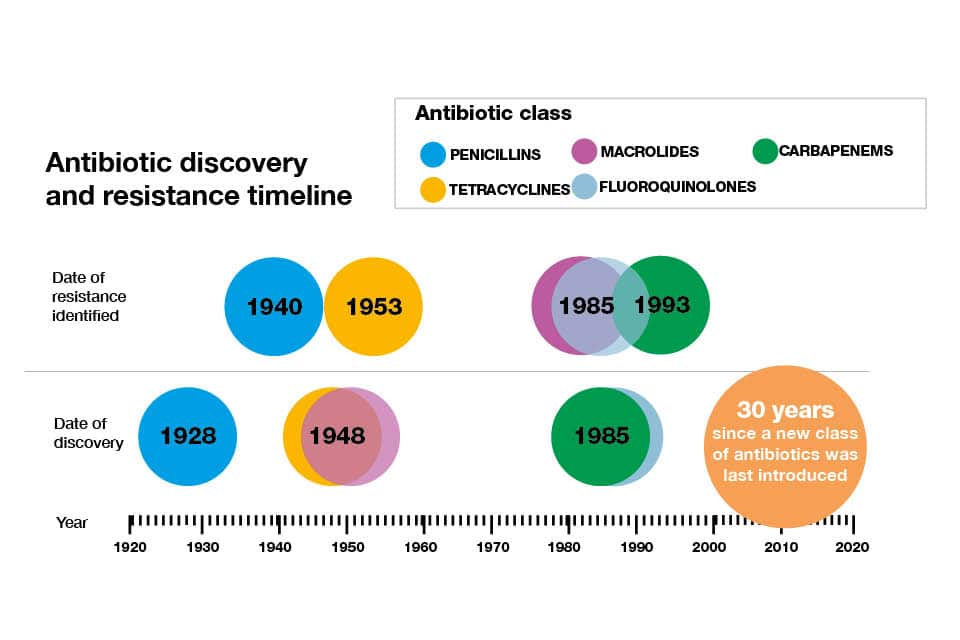
Health Secretary Matt Hancock said: “There is no greater threat to global health than drug-resistant infections, yet there have been no major new antibiotic drug classes discovered since the 1980s. Imagine a world in which a papercut can lead to infection that can’t be controlled. We must stop that from happening. Tackling superbugs needs global leadership and peoples’ lives depend on us finding a new way forward.
“Our NHS is in a unique position to take a global lead in testing new payment models. We will take the lead but this is a global problem and we cannot succeed alone. I am proud the UK is taking the first steps towards a solution and I am urging the rest of the world to join us in the fight against superbugs.”

Health minister Nicola Blackwood
Announcing the project at a speech today at an AMR summit in London, health minister Nicola Blackwood said:
“Having a full pipeline of antimicrobials is critical in our efforts to address AMR, but currently not enough pharmaceutical companies are investing in the development of new drugs.
“This project is an important step but it will only address global market failure if other countries do the same, which is why we want to involve as many countries as we can and share our learning from this work.
“Today we are sending a strong signal to the rest of the world that there are workable models to stimulate investment in these vital medicines and that together we can tackle AMR.”
NICE and NHS England and NHS Improvement are calling for companies to identify products to be considered for the initial phase of the test.
The work will be evaluated from launch and learning will be shared with the rest of the world so that other healthcare systems can also test similar models.
Source: Public Health England
The UK government has also announced £2m for the latest phase of the ‘Keep Antibiotics Working Campaign’,which will be launched in October to help reduce inappropriate use of antibiotics.
Today’s announcements follows the UK’s 2040 AMR vision and 5-year national action plan, published in January, and the appointment of Professor Dame Sally Davies as the first UK Special Envoy on AMR.
Dr Sheuli Porkess, executive director of research, Medical and Innovation at the Association of the British Pharmaceutical Industry (ABPI), said:
“Increased resistance to antibiotics is one of the greatest threats to global health we face.
“Today’s announcement is an example of how the UK can lead the world in this fight and hopefully brings us closer to fixing the problems that have hampered investment in antibiotics research for so long.
“Patients can’t afford to wait. Our members are ready to get started, and the sooner we get this pilot up and running, the sooner we can apply what we find to other antimicrobials in development.”
The ABPI say it “awaits further detail for how the pilot will work in practice,” which individual pharma and biotech companies will no doubt scrutinise. Particular attention will be paid to the size of payments and the conditions attached to them, which will prove crucial to the overall attractiveness of the scheme.
The pilot scheme also reflects a new willingness and capability in the UK to try out new reimbursement models, primarily led by NHS England.
The system could potentially be a boon to many UK-based smaller biotech firms already developing antimicrobials. These include companies such as Bicycle Therapeutics, which earlier this year received a UK government grant to develop a new class of Penicillin Binding Proteins (PBPs) ,in January and Procarta Biosystems, which raised €1.5m in March for its proprietary oligonucleotide-based antimicrobial platform.




