WHITE PAPER: From bench to bedside in one year – how the race for a COVID-19 vaccine will change the future of drug development
July 1, 2021 | COVID-19, drug development, vaccines
In this edition of MAGNIFI, we discuss the factors behind this acceleration in development of the COVID-19 vaccines and what this might mean for the future of pharma
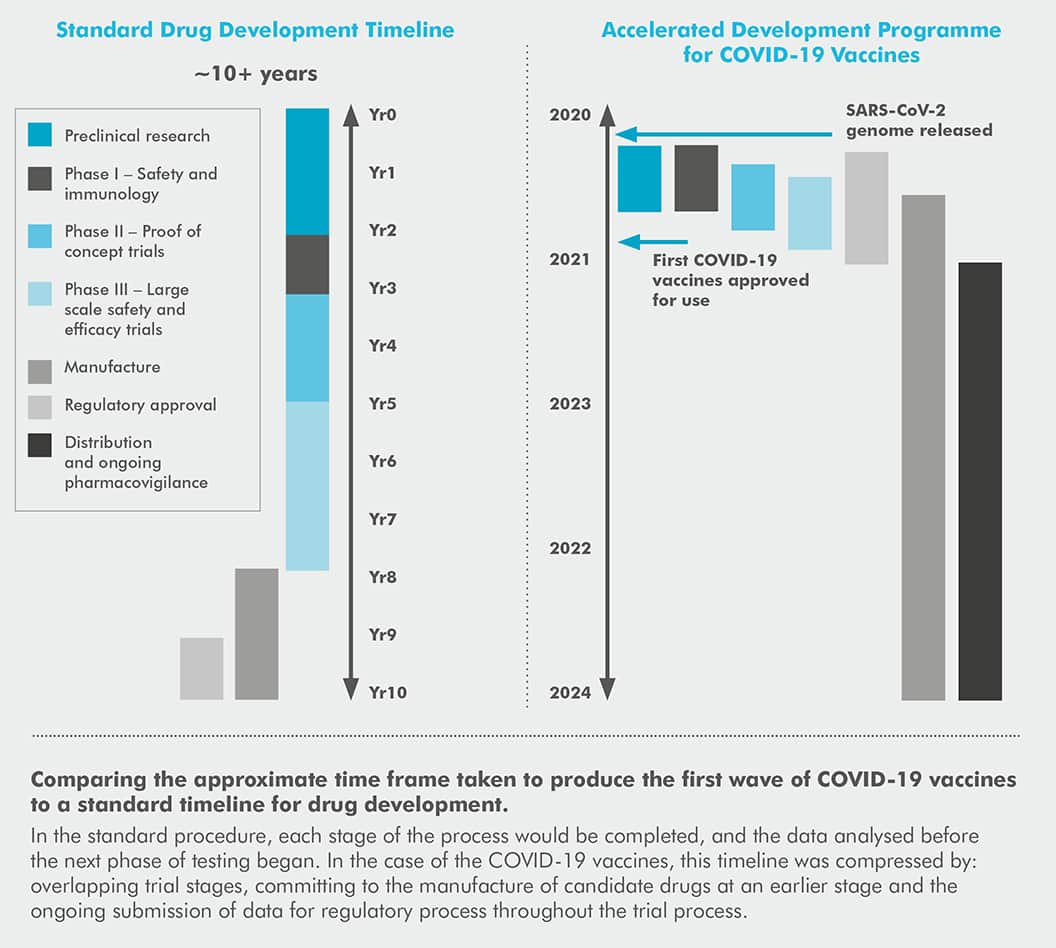
The standard timeline given for developing a new drug from bench to bedside is 10+ years, a figure well-known to the scientific community.
But less than one year after COVID-19 was declared a pandemic, multiple vaccinations were approved for use across the globe. At the time of writing, the vaccination programme in the UK, like many countries, is racing through our adult population with talks of booster jabs being ready for use by the autumn.
In 2020, the onset of the COVID-19 pandemic brought the world to a halt. Almost overnight, we were forced to adapt, undergoing rapid transformation in the way we work, interact with each other, and live our lives.
Although lockdowns and restrictions were able to contain the virus to a
degree, our main hope for a way out of this pandemic was through the development of a successful vaccine that could limit transmission and reduce the severity of infection. However, even if successful, there was no guarantee of a return to normality and with the average time to develop a new vaccine quoted as around 10 years,1 being able to actually produce an effective COVID-19 vaccine was going to be a challenge.
The plan to end the pandemic through the use of vaccines was optimistic, even before taking into account the global disruptions that impacted those working in bioscience like any other sector. Employees were encouraged to work from home where possible, adapting to new, digital ways of working and staff still had to miss work to isolate if they came in contact with the virus.
All of these circumstances make the discovery of over a dozen approved vaccines, along with the introduction of vaccination programmes across the globe in just over a year an impressive feat of science. Within the first four months of our vaccine roll-out, over 10,000 lives are estimated to have been saved in England alone.2
The development of a vaccine was carried out so efficiently in fact, that the time scale to production has even caused members of the public to accuse scientists of cutting corners and taking risks, having the unfortunate effect of negatively impacting public confidence in the vaccine.3,4
By increasing awareness of the reasons behind the efficiency of the world’s efforts to develop a vaccine, hopefully we can restore public confidence that the vaccines were developed in a safe manner and according to industry regulations.
Beyond this, there are lessons to be learnt by both policy makers and the pharmaceutical industry through understanding what the vaccine programmes got right. Hopefully, these lessons will help us to uncover how we can shorten the standard drug development timeline going forward.

12 months to a vaccine: how did it happen?
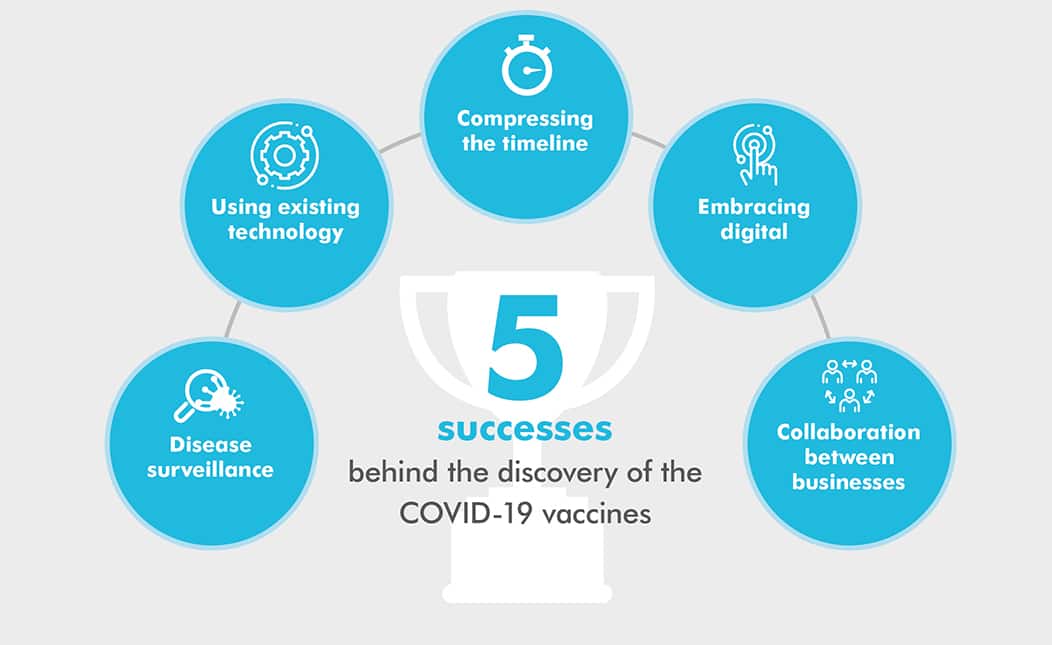
Firstly, it’s important to understand some of the factors that allowed the development of the COVID-19 vaccines to occur in such a timescale:
Disease surveillance gave researchers a head start
Although a common misconception by the public, initial vaccine design and research did not wait until the virus had reached pandemic status to begin.
For years, it has been predicted that a novel virus with pandemic potential could emerge and spread quickly through our interconnected society. Therefore, scientists and public health authorities were already carrying out surveillance in preparation, ready to start research into a vaccine for any concerning viruses that might emerge.
One group involved in this area of research was the team at Oxford University, who’s efforts eventually became the Oxford-AstraZeneca vaccine currently in circulation. This research team began work on their vaccine design at a time when the only indications that the virus had pandemic potential were in its DNA and observed characteristics.5
Vaccine design could be based on an existing template
It’s also important to realise that the vaccines developed over the past year are not entirely new technologies, and while the COVID-19 vaccines are the first approved medications to use mRNA vaccine technology and among the first to use viral vectors,6,7 years of research and funding had
already been invested into these techniques.8 This meant that the technology could be applied to new targets once enough was known about the novel coronavirus.9
The team at Oxford were using the viral vector approach, which they had previously used to make candidate vaccines in outbreaks of flu, Zika and Middle East Respiratory Syndrome (MERS).5 This meant that they had the framework for the vaccine, allowing them to produce their initial vaccine design within 48 hours of receiving the full genetic sequence of the virus.10
To put this into context, the Oxford team began their vaccine design the day before the first death attributed to this emerging virus would be recorded in China, and a month before the virus would even be given a name.11,12

The uptake and investment in digital technologies surged
With research underway and data accumulating, this was another point where modern technologies were able to benefit the vaccine development process. Over the past year, we have embraced digital in our home lives at an unprecedented pace. The same can be said for the pharmaceutical industry, with experts suggesting that biopharma has progressed further in the past 10 months than in the previous 10 years.13 Based on necessity, companies invested in digital processes that would improve the ease of both communication and data analysis, which greatly benefited vaccine research.13
Now that the focus is on the global deployment of the vaccines, it is hoped that this is also an area that can be simplified by integrating digital approaches. For example, the Medicines and Healthcare Products Regulatory Agency (MHRA) have invested in efforts to use artificial intelligence (AI) in order to improve the process of reporting adverse events, which hopefully could help to decrease vaccine hesitancy.14
Data processing capabilities will also be useful for processes such as tracking vaccine stock, counting the number of patients who have received the vaccine and monitoring the temperature of vials to ensure safe transport and storage.15 However, the uses of digital will depend on variations in data protection laws and the existing infrastructure for digital capabilities present within countries.15
Concurrent trials and bold decisions by investors allowed the timeline to be compressed
Under normal circumstances, each phase of a drug trial would be carried out one after the other, with review processes and investment decisions adding increased delays during this time.8 However, in the case of COVID-19, speed was essential, and a revised clinical testing schedule was created. This revised schedule was based on previous efforts to accelerate the timeline that occurred during the Severe acute respiratory syndrome (SARS) and MERS outbreaks of the past 20 years.8
One big change that allowed the development timeline to be shortened was the decision to overlap Phase I, II and III clinical trials, all while scaling up the manufacturing processes for multiple candidates.16 This facilitated a decrease in the overall time each candidate spent in the clinical trial phase alongside ensuring that the vaccines were ready to distribute as soon as regulatory approval was given.16
Another important factor that had a big impact on the timeline was that pharmaceutical and biotech companies invested in the manufacturing capabilities of promising candidate vaccines much earlier in the development process and with less information available than usual to help inform their decisions.8 These companies took a great financial risk in order to keep delays in the development process to a minimum and to play a part in vaccinating the world.
Companies collaborated for a common goal
With the common goal of global vaccination and an enormous challenge ahead of them, competitors became allies in order to progress the vaccine efforts. In the past year, we have seen partnerships between companies such as Pfizer and BioNTech; Oxford University and AstraZeneca and more recently GSK and CureVac. Industry players also worked together with external organisations such as governments and regulatory bodies in order to streamline the development process.17
Going forward, collaboration will be essential in order to deploy vaccines across the world and is why the World Health Organisation (WHO) are pushing for a global approach towards ending COVID-19 and avoiding vaccine nationalism.18 With the risk of the emergence of new, vaccine evading mutations growing greater as transmission continues, vaccinating all countries is essential if we want to avoid backsliding.
All countries will require access to vaccines, regardless of wealth, so collaborative efforts such as the COVAX scheme (an initiative set up to provide access and funding for vaccination programmes across the globe) are a crucial investment.
Are we in a better place to deal with the next crisis?
What have we learnt?
Despite the predictions, at the start of the pandemic much of the world was caught off-guard by the emergence of COVID-19 and unable to prevent initial community spread.
There is a lot that can be learned from our shortcomings to help us be more prepared for the next health crisis. Some of the weaknesses identified during the pandemic that we should start to address include:
- The capacity of our health services19
- Global disease surveillance and response systems20
- Complacency when it comes to the prevalence of chronic health conditions in the general public21
- Discrepancy in health outcomes for communities of different ethnicities and socioeconomic backgrounds22,23
- Public trust in their government, science, and the safety of vaccination21
This final point will be a key issue going forward. While global vaccine efforts have been a huge success, a lack of public trust and understanding of the drug development process have been a challenge throughout all stages of this process.
From scepticism around how quickly the UK approved the first vaccine24 to concerns around the safety of the Oxford-AstraZeneca jab, mixed messages from scientists, healthcare professionals and politicians have all left a dent in public confidence.25 A crisis in confidence had the potential to derail the whole vaccination plan, which relies heavily on a sufficient uptake of the vaccines within the population. Going forward, this trust will need to be rebuilt.
What can we carry forward?
Hopefully, like the lessons learned from the SARS and MERS outbreaks of the past 20 years, our experience with COVID-19 will help us to be better prepared for the next health crisis, whatever that may be.
In the decades preceding the emergence of COVID-19, the scientific community warned of the emergence of a virus with potential to be the next Spanish Flu. However, when it did come along the correct precautions were not in place. So, what else are scientists warning of?
The next health crisis may not be another infectious disease that we can address with vaccines. For years, concerns have been mounting about a problem that has the potential to set our health services back 100 years: the rise of antimicrobial resistance.
More and more bacterial infections that would previously have been treatable are proving resistant to multiple types of antibiotics.26 Making this situation more concerning – the antibiotic discovery pipeline has dried up. Due to the nature of this issue, any newly discovered antibiotics would be designed to be kept in reserve for emergencies, so antibiotic discovery is not a particularly profitable field.27 However, it is currently predicted that if we don’t act on this issue now, we could be seeing 10 million deaths related to drug resistant infections per year by the year 2050.28
Addressing this problem will require:
- Proactivity before the issue becomes a full-blown crisis
- Public understanding and cooperation when it comes to responsible antibiotic use
- Risk taking and investments from governments and pharmaceutical companies for the benefit of global health
These are all lessons that we have had to learn over the course of the pandemic. Could COVID-19 leave us better prepared for the bigger crisis waiting around the corner?
What does this mean for the future of drug development?
In the coming months and years, it will be interesting to see which of the processes adopted when developing the vaccines will become more commonplace in day-to-day drug development.
While certain changes, such as the bold and risky decisions made by pharma and biotech companies, enormous investments of both time and money by all involved and ‘fast-tracking’ through the regulatory process are likely special cases, some changes could be here to stay:
The use of mRNA and viral vector vaccines could become more commonplace
Both of these technologies have benefited greatly from the vaccine race, with a large quantity of clinical trial data and real-world evidence generated to support their safety and efficacy for use. While they have both been hot topics in the industry for a while, they have undoubtedly been fast tracked toward more common use, with trials for the use of mRNA vaccines to prevent and treat HIV infection already underway.8,29–31
Digital is here to stay
The need for social distancing has furthered the development of technologies that allow elements of the research and development process to be carried out remotely. One of the big changes we have seen over the past year is the rise of decentralised trials.32 These are trials that are carried out largely in the patient’s home with the help of telemedicine and local healthcare providers. It is thought that they have the potential to increase trial participation by making the whole process more flexible and convenient for patients.32 The rise in decentralised trials has been facilitated by technologies, such as AI, machine learning and cloud computing.32
We have learned the benefits of collaboration
Over the past year, partnerships between competitor companies and across industries have allowed the world’s best minds to work together towards an end goal. By demonstrating what these collaborations can achieve, we have highlighted the unmet potential in other therapeutic areas along with the inefficiency of the current system.
Alongside the partnerships we are seeing between small tech start-ups or universities with innovative ideas with the large companies that have the means to translate those ideas to market, will we start to see pharma companies think more broadly when it comes to their next collaborations?
Will we see a return to the 10+ year development timeline?
Although the urgency of the pandemic benefitted the vaccine efforts with an unprecedented amount of funding, data and time from stakeholders across the globe, it’s hard to see the process of drug development returning to its previous state.
Without the usual resource-based limitations, the scientific community was given the freedom to completely rethink how we approach the trial process in light of the technology and capabilities we have today, which has ignited a new wave of innovation. Once the public health threat of the pandemic has been mitigated, we will really start to see the impact of this new way of thinking.

References:
1. Mullard A. COVID-19 vaccine development pipeline gears up. Lancet. 2020. doi:10.1016/S0140-6736(20)31252-6
2. Andrews N, et al. Impact of COVID-19 Vaccines on Mortality in England. London; 2021.
3. Torreele E. The rush to create a covid-19 vaccine may do more harm than good. BMJ. 2020;370. doi:10.1136/bmj.m3209.
4. The big question over a Covid vaccine: Is it coming too fast to be trusted? The Independent. https://www.independent.co.uk/news/world/americas/coronavirus-vaccine-phase-3-covid-fda-cure-a9704111.html. Published 2020. Accessed May 26, 2021.
5. About the Oxford COVID-19 vaccine. University of Oxford. https://www.research.ox.ac.uk/article/2020-07-19-the-oxford-covid-19-vaccine. Published July 19, 2020. Accessed June 10, 2021.
6. Komaroff A. Why are mRNA vaccines so exciting? Harvard Health. December 2020. https://www.health.harvard.edu/blog/why-are-mrna-vaccines-so-exciting-2020121021599. Accessed June 10, 2021.
7. Understanding Viral Vector COVID-19 Vaccines. CDC. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different vaccines/viralvector.html. Published April 13, 2021. Accessed June 10, 2021.
8. Agrawal G et al. Fast-forward: Will the speed of COVID-19 vaccine development reset industry norms? McKinsey. https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/fast-forwardwill-the-speed-of-covid-19-vaccine-development-reset-industry-norms. Published May 13, 2021. Accessed June 2, 2021.
9. Ball P. The lightning-fast quest for COVID vaccines – and what it means for other diseases. Nature. 2021;589(7840):16-18. doi:10.1038/d41586-020-03626-1
10. Walsh F. Oxford-AstraZeneca vaccine: Bogus reports, accidental finds – the story of the jab. BBC News. https://www.bbc.co.uk/news/health-55308216. Published December 2020. Accessed June 2, 2021.
11. Neilson S, et al. Coronavirus: a 1-Year Timeline of the Pandemic Since China’s 1st Case. Insider. https://www.businessinsider.com/coronavirus-pandemictimeline-history-major-events-2020-3?r=US&IR=T. Published December 24, 2020. Accessed June 10, 2021.
12. COVID-19 vaccine development. Oxford Vaccine Group. https://www.ovg.ox.ac.uk/news/covid-19-vaccine-development. Published March 18, 2020. Accessed June 10, 2021.
13. Agrawal G, et al. The biopharma industry has shown what it can achieve when it works at its best. How can the industry build on this renewed sense of purpose in the years ahead? McKinsey. https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/biopharma-2020-a-landmark-year-and-a-reset-for-the-future. Published January 8, 2021. Accessed June 2, 2021.
14. Can technology increase COVID-19 vaccination rates? Lancet Digit Heal. 2021;3:e274. doi:10.1016/S2589-7500(21)00061-3.
15. Eichholtzer M, et al. Digital technologies can support countries to face the scale and complexity of COVID-19 vaccine delivery. World Bank Blogs. https://blogs.worldbank.org/digital-development/digital-technologies-can-supportcountries-face-scale-and-complexity-covid-19. Published 2021. Accessed June 2, 2021.
16. Saborni Chakraborty,et al. SARS-CoV-2 vaccines in advanced clinical trials: Where do we stand? Adv Drug Deliv Rev. 2021;172:314-338. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7816567/ Accessed June 2, 2021.
17. Byrne J. Key learnings from COVID-19 vaccine development: Power of cross industry collaboration and need for ethnic diversity in clinical trials. Biopharma Reporter. https://www.biopharma-reporter.com/Article/2021/02/10/Key-learnings-from-COVID-19-vaccine-development-Power-of-cross-industry-collaboration-and-need-for-ethnic-diversity-in-clinical-trials. Published February 10, 2021. Accessed June 2, 2021.
18. Eaton L. Covid-19: WHO warns against “vaccine nationalism” or face further virus mutations. BMJ. 2021;372:n292. doi:10.1136/bmj.n292.
19. Care Quality Commission. The State of Health Care and Adult Social Care in England 2019/20.; 2020. www.gov.uk/official-documents. Accessed June 21, 2021.
20. Carroll D, et al. Preventing the next pandemic: the power of a global viral surveillance network. BMJ. 2021;372:n485. doi:10.1136/bmj.n485.
21. Preparing for the next pandemic. Nat Med. 2021;27(3):357. doi:10.1038/s41591-021-01291-z.
22. Johnson-Mann C, et al. COVID-19 pandemic highlights racial health inequalities. Lancet. 2020;8:663-664. https://doi.org/10.1016/ S2213-8587(20)30225-4%0A.
23. Paremoer L, et al. Covid-19 pandemic and the social determinants of health. BMJ. 2021;372. doi:10.1136/bmj.n129.
24. Dean G, Reuters. The UK approved Pfizer’s vaccine too quickly and without the proper checks, EU politicians have warned. Business Insider. https://www.businessinsider.com/uk-approved-pfizer-covid-19-vaccine-too-quickly-eupoliticians-2020-12?r=US&IR=T. Published December 2, 2020. Accessed June 10, 2021.
25. Co-creator of AstraZeneca COVID shot defends safety amid clot concerns. Reuters. https://www.reuters.com/business/healthcare-pharmaceuticals/co-creator-astrazeneca-covid-shot-defends-safety-amid-clotconcerns-2021-04-23/. Published April 23, 2021. Accessed June 10, 2021.
26. Antimicrobial resistance. WHO. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Published October 13, 2020. Accessed June 10, 2021.
27. McKenna M. We Need Antibiotics. They’re Not Profitable To Make. Who Pays? Natl Geogr Mag. May 2015. https://www.nationalgeographic.com/science/article/oneill-amr-3. Accessed June 10, 2021.
28. Hutchings M, et al. Antibiotics: past, present and future. Curr Opin Microbiol. 2019;51:72-80. doi:10.1016/j.mib.2019.10.008.
29. Kwon D. The Promise of mRNA Vaccines. Sci Mag. November 2020. https://www.the-scientist.com/news-opinion/the-promise-of-mrna-vaccines-68202. Accessed June 10, 2021.
30. King A. Vector-Based Vaccines Come to the Fore in the COVID-19 Pandemic. The Scientist. https://www.the-scientist.com/news-opinion/vector-basedvaccines-come-to-the-fore-in-the-covid-19-pandemic-67915. Published September 28, 2020. Accessed June 10, 2021.
31. Mu Z, et al. HIV mRNA vaccines- progress and future paths. Vaccines. 2021;9(2):1-22. doi:10.3390/vaccines9020134.
32. Wosiack, C R. Decentralised clinical trials: paving the way for modernisation. Eur Pharm Rev. April 2021. https://www.europeanpharmaceuticalreview.com/article/149375/decentralised-clinical-trials-paving-the-way-for-modernisation/. Accessed June 10, 2021.





