Seen as the future of pharma for many years, cell and gene therapy (C>) is finally coming of age – advances in technology and new commercial viability mean we are closer than ever to that future becoming reality.
The result is greater levels of investment in the companies that are leading the way forward, along with an environment that is ripe for M&A consolidation, with larger pharma companies eager to prevent start-up innovators from gaining an unassailable head start in such an important field.
Firstly, it is important to understand exactly what cell and gene therapy is, and why, given its potential has long been known, it is only now becoming the centre of attention for the main pharmaceutical players. Often lumped together as terms, the key difference between cell therapy and gene therapy is that the former utilises cells from a donor or the patient themselves to treat a disease through their modification and insertion (or re- insertion) into the blood stream.
By contrast, gene therapy involves modifying, copying or replacing a gene. Recent innovative techniques that can reach genes without causing significant side effects have triggered a surge in activity in the area.
The reason both therapies are linked together so regularly is due to their crossover: both involve targeting specific genetic characteristics. Cell therapy often strays into gene therapy territory when it is decided that an easier method to treat cells would be to extract them from the body and rework their genetics.
Such complex solutions were, understandably, seen as too advanced, risky, or frankly not commercially viable enough. But with continued innovation – much of it coming from biotech companies – we have now reached the stage where products once on the periphery of pharmaceutical research are being sanctioned with FDA approval, with the organisation expecting to approve between 10-20 C> products a year by 2025.
M&A activity surging
With large pharmaceutical companies reluctant to miss out on such an important advance in medicine, M&A activity in the space has increased exponentially. In 2014-15, M&A deal values in this sector were $5bn, and in 2018-19, this surged 980% to a combined two-year total of $49bn. The cell therapy, gene therapy and tissue engineering sector raised $2.9bn in venture capital in 2018 – twice the 2017 amount.
Recent examples of this trend can be seen in Roche’s December 2019 $4.8bn takeover of Spark Therapeutics, one of the first US gene therapy firms to succeed in obtaining FDA approval – in this case for a project that can treat inherited vision loss. Another example is Biogen’s $800m acquisition of Nightstar’s late-stage gene therapy assets for retinal disease – NSR-REP1 and NSR-RPGR.
Both deals represent well- capitalised pharma giants paying large sums for completed, or nearly completed, final products, giving the buyer an almost guaranteed product to start manufacturing, branding and distributing immediately. Such deals often benefit all parties involved – sellers may well have been struggling under the costs to pursue trials and approvals (even for extremely promising candidates) and would have faced extreme financial pressure in trying to bring a treatment to market.
Pharma’s next wave of innovation, yet challenges remain
C> has innovated at such a rapid pace that much of this appetite for M&A is also led by a hangover of big pharma being left behind in the sphere of monoclonal antibody technology. Then, pharma firms did not react in time and lost ground to advancing smaller firms. Now, these companies are aiming to give themselves ample opportunity to acquire, partner and strengthen their hand in the C> space.
Indeed, with treatments already at approval stage, market revenue is close at hand. With the nature of C> being highly tailored and often one-off, it represents a new form of revenue generation that can command high prices for each treatment, rather than the gradual revenue obtained from conventional drug sales.
Such a model also presents the flourishing sector with challenges. High prices can cross into financially unviable territory, with a prime example being the innovative treatment Zolgensma. The one-off therapy, which delivers a new, working SMN gene to the motor neurone cells in the
body to target motor neurone disease, is FDA approved and developed by Avexis, but it won’t see widespread availability due to a price tag of $2.1m per treatment.
Projects that require such tailored solutions are harder to manufacture than traditional medication, and another challenge arises in the form of medical providers’ payment preferences. Traditionally, hospitals are more comfortable with paying for constant, easily affordable medication; C> presents them with a high upfront cost, albeit one they rarely have to repeat on the same patient.
However, continued innovation shows progress can be made in solving these problems. In addition, the continued success of C> may prompt reworked financial models as it becomes more established in the industry.
The argument for continued investment
C> is a high potential and maturing sector, and is an already crowded environment, playing host to numerous start-ups and now, through M&A, recognised big pharma firms. Much like the rush to find a COVID-19 vaccine that dominates headlines worldwide, not every company involved will be able to succeed.
But finnCap’s finnLife watch list of 50 leading AIM-listed biotech companies demonstrates that there is room for numerous companies to contribute to, and profit from, C>. Examining three entirely different approaches to CAR-T therapy, it is possible to see just how much space there is for this exciting sector, therefore displaying the case for continued investment.
Innovative CAR-T therapy demonstrates the depth of C> potential
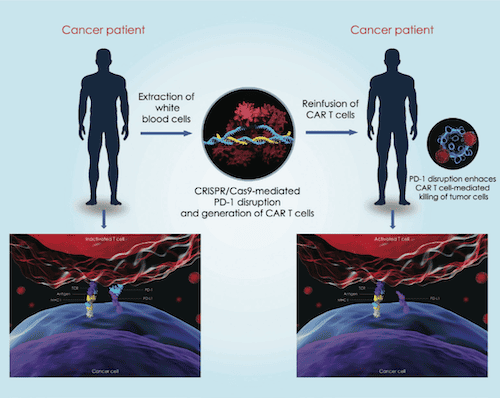
CAR-T therapy in its existing form is a relatively new and specialised approach at treating cancer. It takes T cells from a patient’s bloodstream and genetically modifies them in a laboratory. These T cells are then injected back into the bloodstream with the aim of targeting and killing cancer cells.
While it has been shown to be an effective treatment, there are risks and side effects. One is the two-step autologous process (the slow time it takes for cell expansion – sometimes as long as two weeks) while another is cytokine release syndrome (CRS), which occurs when cytokine molecules are inadvertently released, but too quickly to target just the tumours and instead target healthy cells.
The next generation of CAR-T treatments shows that there is space for a multitude of start-ups to be active in the C> space as they all help find varied solutions to these problems without negating the effectiveness of CAR-T.
One example is Horizon Delivery, a company that is developing its CYAD-02 project, which will help transport T cells more effectively to the tumour via the use of SMARTvector products.
The product underwent its first phase 1 trial test in January 2020 with a patient who was suffering from acute myeloid leukaemia. Horizon Delivery is also an industry leader in CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) screenings, meaning they can identify key genes or genetic sequences that draw out specific functions of a cell type from thousands of potential variants.
In a cancer context, this means they can route out and exclusively eliminate problematic cells that may have shown signs they’d resist a future cancer treatment.
Another example is Maxcyte, a global cell- based therapies and life sciences company that is developing its CARMA process, where a patient’s peripheral blood mononuclear cells (PBMCs) are removed and modified. The modified cells can then be used to target an array of different cancers.
Currently the company is conducting a phase 1 trial for advanced ovarian cancer in a dose escalation trial that will treat four separate cohorts – the fourth of which was administered in March 2020.
Another example which shows the versatility of new CAR-T innovation is provided by Oxford Biomedica, a gene and cell therapy company specialising in the development of gene-based medicines.
Rather than a contained project or platform, its contribution to CAR-T is through a contract manufacturing development organisation. Collaborating with pharma companies, Oxford Biomedica uses its infrastructure to produce other companies’ licensed products, including Novartis’ Kymriah treatment (alongside other undisclosed CAR-T-related products).
With fast-moving innovation finally allowing multiple C> treatments to gain regulatory approval, along with a huge pipeline of upcoming therapies and an influx of funding and M&A activity, investing in C> no longer entails taking a bet on potential – the future is finally here.




