


The UK is undergoing a seismic shift in healthcare strategy, away from a primary focus on treatment of ill health, towards one of prevention and overall population health, a trend fortified by the NHS Long Term Plan. It is happening at pace, driven by a unique combination of factors including Brexit, COVID-19 and NHS reforms. The implications for pharma industry market access are significant, requiring rapid and fundamental adaptation in the face of a fast-changing environment.
The good news for pharma is that these changes bring opportunities, and – with the pandemic having enhanced the industry’s reputation and proved the need for investment and collaboration – the industry is now viewed by the Government and NHS leaders as a fundamental partner. There is an openness to disruptive innovation that perhaps was not there before.
It is in this context that ‘smart deals’ have emerged in recent years – these are deals involving a clinically led procurement approach for innovative medicines that address critical unmet needs, offer a population-health benefit, and/or support the delivery of wider NHS priorities. For pharma, smart deals can offer expedited market access to innovative medicines – often in return for discounts and a commitment to collect real-world evidence (RWE) for evaluation by the National Institute for Health and Care Excellence (NICE). So, what do these deals look like in practice? And what is the likelihood they will start to appear in other countries beyond the UK?
A perfect environment for innovative market access
It is important to first acknowledge the extent to which the UK market access environment has transformed – and why it is now ideal for smart market deals. Back in 2008, faced with innumerable hurdles to product launches in the UK, the then chief executive of Novartis UK announced there was “a real possibility that drug companies will stop launching new medicines in the UK”.
Fourteen years later, the UK is now leading the way in market access solutions for innovative therapies. The Life Sciences vision – intended to build on the successes of the COVID-19 response and accelerate the delivery of innovations to patients – reaffirms the NHS Long Term Plan’s narrative of the need for prevention, population health, value and better management of chronic conditions.
Simultaneously, other recent initiatives have helped to create a supportive environment for father market access. The Innovative Licensing and Access Pathway (ILAP) was introduced to accelerate time to market for innovative medicines, and Project Orbis provides a framework for concurrent submission and review of oncology products among international partners.
Leqvio leading the way?
It is perhaps no wonder then that the momentum for smart deals is growing. In 2021, 16 pricing and access deals were struck with the NHS, ten of which can be considered ‘smart’ – a new record for the UK, made even more impressive against the backdrop of the pandemic. Perhaps the most widely known of these was the deal struck with Novartis for access to its novel cholesterol drug, Leqvio (inclisiran).
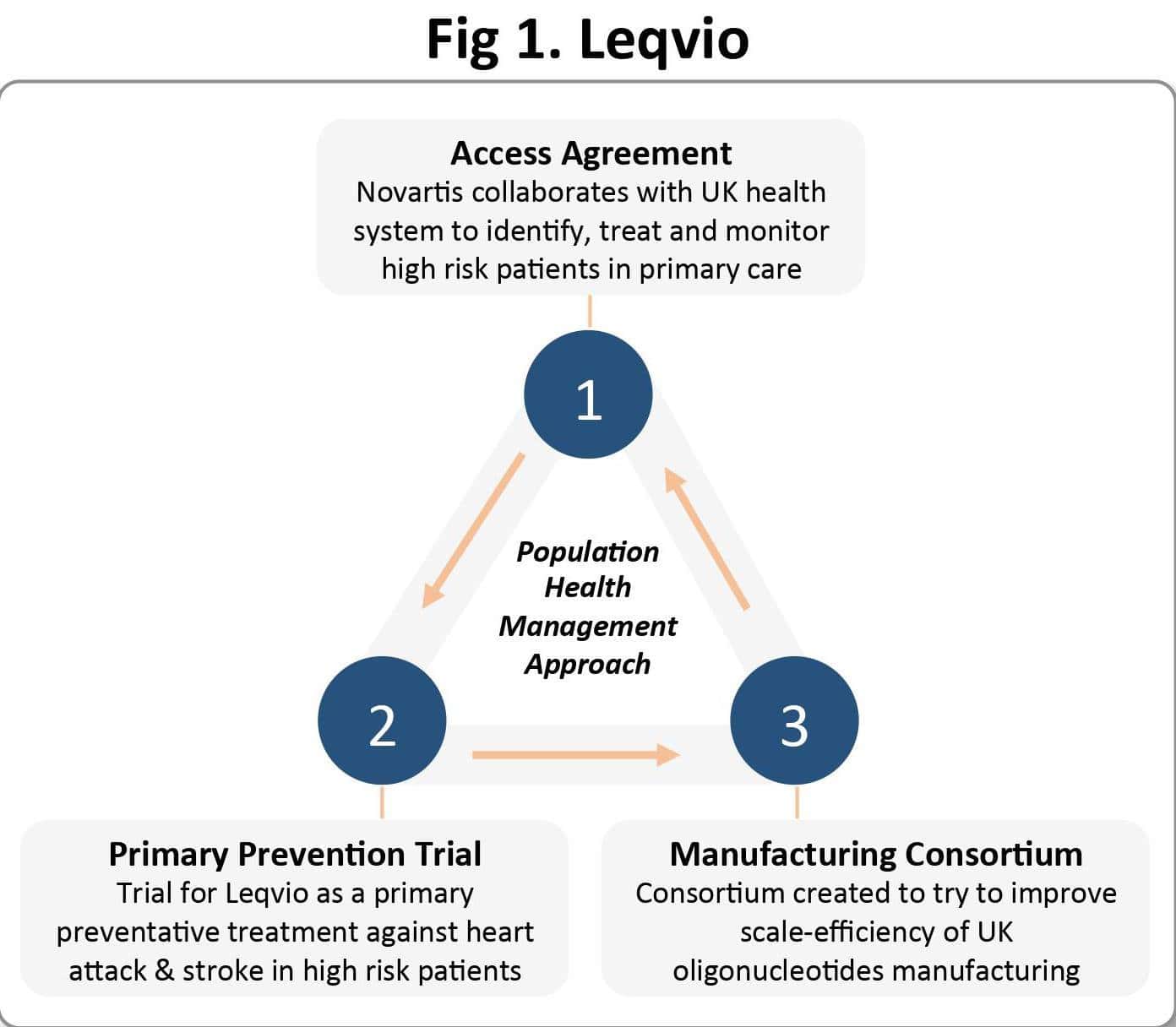
It represented a first-of-its-kind population health management approach to address elevated low-density lipoproteins cholesterol (LDL-C) in eligible patients across England. There are around 7.6 million people living with cardiovascular disease (CVD) in the UK, and the NHS Long Term Plan identifies CVD as the single biggest condition where lives can be saved by the NHS over the next ten years. Driven by its new population health strategy, the NHS entered the smart deal with the hope of reducing the estimated 100,000 hospital admissions each year and saving an estimated 30,000 lives within the next decade.
There are three integral elements of the deal (see Figure 1): the access agreement, which specifies that Novartis will collaborate with the NHS to proactively identify, treat and monitor individuals in the primary care setting who are ‘at highest risk’; a large-scale primary prevention trial of Leqvio; and the creation of a consortium with academic groups aimed at improving the manufacture of oligonucleotide drugs in the UK.
Smart deals already proving their value
In theory, smart deals like this are win-win-win. Patients receive access to essential therapies. The health system moves towards its goal of greater population health and saves money in the process – indeed, the NHS recently claimed that ‘smart deals’ have saved taxpayers £1.2bn on medicines procurement over three years. And pharma companies enjoy expedited market access with greater opportunities for the future.
Take Vertex for example, with its cystic fibrosis (CF) drug Kaftrio, which significantly improves lung function and overall quality of life for patients with CF. In 2020, the NHS agreed a ‘landmark deal’ with Vertex (see Figure 2), enabling immediate access to the transformative drug, as well as others within Vertex’s CF portfolio that target rare mutations, under the condition of additional RWE collection for NICE reassessment.
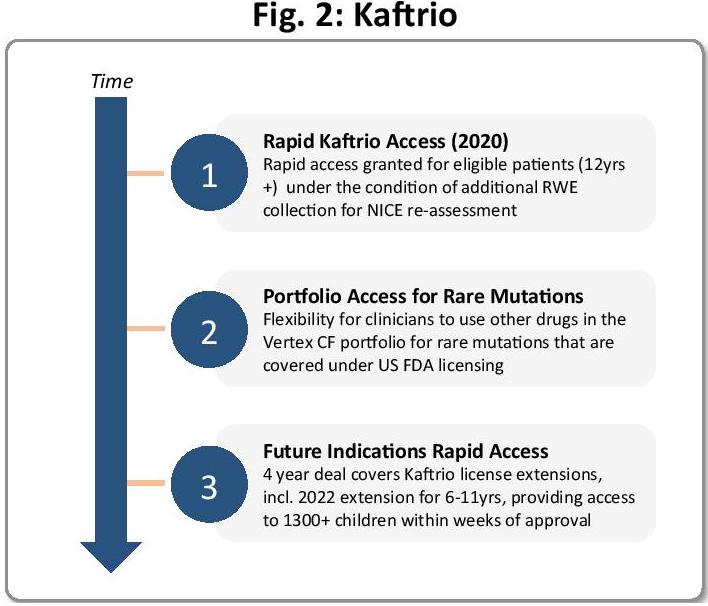
Thanks to the smart deal, the UK roll-out of Kaftrio was one of the fastest in the world. Two years later, Vertex received an extended licence for Kaftrio, for six- to 11-year-olds, with the potential to reach over 1,300 new patients in the UK with significant unmet need. Under the terms of the 2020 deal, immediate access to those newly eligible patients was ensured, and the value provided to the CF community extended significantly.
Such deals also provide an excellent foundation for RWE generation, which is invaluable for both health systems and the industry to evaluate outcomes and assess value.
To support this further, NICE has recently published its Real-World Evidence Framework, a ‘living document’ to facilitate best practice for planning, conducting and reporting RWE, to improve quality of RWE used to inform NICE and increase overall trust in RWE studies.
To ensure patient access is possible while RWE is collected, the Innovative Medicines Fund was established in 2021 – following the success of the Cancer Drugs Fund – to provide access to promising new medicines while more data is gathered. For pharma, this enables expedited access via alternative funding, along with the opportunity to gather additional evidence to address any uncertainties regarding clinical and cost- effectiveness.
So far so good, but there may still be bumps in the road. If we look again at Leqvio, the deal heralded as a ‘game changer’ is not quite reaching those heights yet. There are several hurdles to be surmounted – particularly in expanding the underlying trial data set and building delivery capability in primary care. With a restructure of local lipid management pathways required, as well as training of primary care physicians on administration of injectables – all while there is an ongoing patient backlog within primary care caused by the pandemic – it is easy to see why a national strategy does not always immediately translate to implementation on the ground.
The smart way to go in the future?
Despite the hurdles seen in the Leqvio case, smart deals present an opportunity for all stakeholders involved, providing expedited access to innovative medicines, structured opportunities for evidence collection and real progress towards common healthcare system priorities such as preventative, population health.
With that in mind, it seems likely other countries will follow the UK example before long, particularly given the progress already made towards successfully implementing value-based agreements in countries such as Spain and Italy. The trends towards value-based healthcare and population health are happening all over the world. And with the explosion of scientific innovation and digital technology in recent years, the need to utilise RWE is greater than ever and more implementable.
For pharma companies, the onus is on them to be open to these deals and to prepare accordingly. What does that look like in practice? Well, companies need to show a willingness to collaborate early and continuously with health systems and, vitally, demonstrate that the value of their new therapy is in line with the strategic focus areas of the health systems. This value can be further established via RWE, and, in the meantime, the company should demonstrate flexibility in terms of pricing, with the pay-off of faster access and potentially expanded opportunities further down the road.
Clearly there are extra steps and extra thinking involved when considering smart deals, but the benefits to those who are willing and able to be innovative could be significant.
It is a fast-changing environment. And the precise path other countries will take is unknown. But, given the similarity of challenges and opportunities in healthcare all over the world, the situation in the UK is likely to be replicated in other countries in the years ahead. This makes early engagement with this approach the smart thing to do.




