We’ve all heard the saying: what gets measured, gets done.
The merits of the argument are well debated, but there can be no denying that measuring and benchmarking progress are key to commercial success. So here’s the thing: what if you can’t measure something that’s vitally important? What if you can’t see what’s actually happening in that crucial area, to understand what it means for your brand and respond accordingly?
Flying blind is dangerous. Yet that’s exactly what many pharmaceutical companies do when it comes to measuring a primary driver of product sales: formulary adoption. They monitor it, they pursue it and they set targets to achieve it; but they cannot truly see it. It’s a missed opportunity. This article explains how, with good formulary intelligence, you can double your sales in the first year of launch.
Competitive advantage
Relative formulary status is the gateway (or the barrier) to market access. Over the years, pharma companies have used it as a baseline indicator of performance measurement – but its value is so much greater.
In-depth (and agile) understanding of formulary dynamics – not just for your brand but for the whole competitive environment – can inform a range of important commercial decisions: from strategic planning, budgeting and resourcing, to customer targeting and differential messaging.
What’s more, in an age when pharma’s operating models are being transformed by digital innovation, a good pulse on the formulary landscape can drive an evidence based approach to multichannel engagement; right customer, right message, right time, right channel, right frequency.
We all know the story – it’s time to rewrite the ending. In fiercely competitive markets where everyone’s crying out for a USP, good formulary intelligence can be a powerful differentiator. However, at present the opportunity is largely unfulfilled. Too often, companies’ lens on the formulary landscape is primitive; they rely on old-fashioned snapshots of yesterday, rather than the wide-angle view of the bigger picture that increases their prospects of commercial success.
Formularies are always evolving, so moment-in-time snaps are quickly out of date. To fill the void, organisations typically ask their field-based teams to track and report formulary updates. It’s understandable but flawed. Field teams work at the coalface and have a close-up view of the customer’s world – but capturing formulary data can, at best, distract focus from their primary objectives and, at worst, mean important nuances are overlooked.
The formulary landscape is messy and complex. Tracking it is not just about pure numbers – it’s about standing back from the information, understanding it in the context of the competitive environment and working out what it means for access to your brand. In the helter-skelter of everyday stakeholder engagement, field teams rarely have the time or the tools for detailed evaluation.
Instead, many end up compiling a basic scoreboard of formulary status that doesn’t reflect the complexities behind the locality legends, doesn’t interpret formulary intent and doesn’t help them tailor differential strategies for individual localities. ‘What gets measured, gets done’ – but it could all be done much more effectively, empowering brand teams with better information that stimulates competitive advantage.
Lessons from lockdown
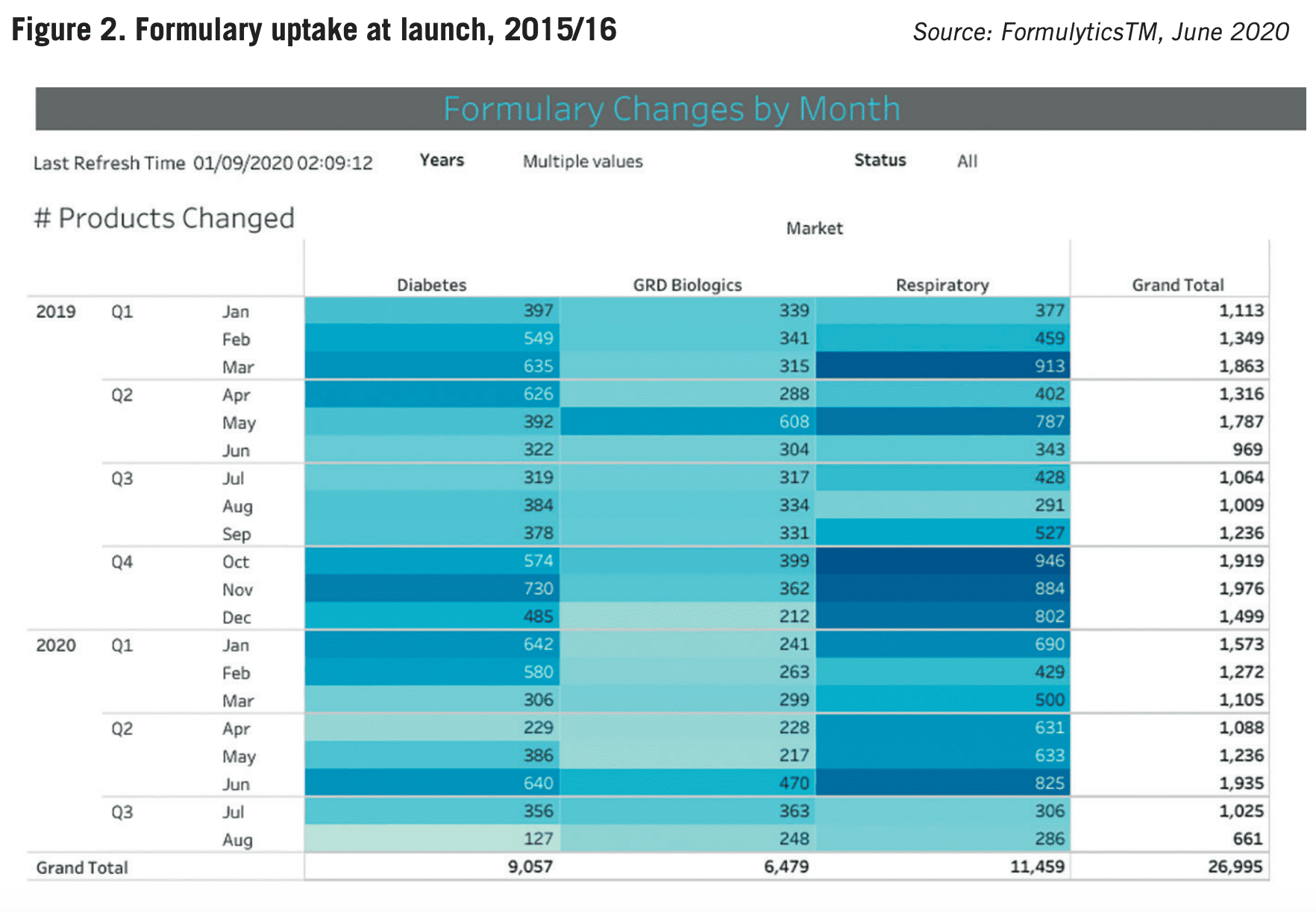
The limitations of relying on the field force for formulary intelligence have been brutally exposed by COVID-19. The enforced absence of face-to-face engagement has meant that many companies have been blind to what’s happening on the ground. However, despite the widespread redirection of healthcare resources to focus on managing the coronavirus emergency, formularies have continued to update and change throughout the pandemic.
Longitudinal research of NHS formularies in three major therapy areas shows that the volume of change in the first two months of lockdown maintained the same levels seen 12 months earlier. But the numbers are only the start of the story; it’s not enough to know that something has changed, you need to understand what it means and how it shapes the market for your brand. Significant clues for brand planning, customer segmentation and differential resourcing are hidden away in formulary data – but only if you’ve got the wherewithal to spot them, interpret them and plan around them.
Launch expectations: the importance of formulary intelligence
Experience suggests that those who track change and evaluate trends tend to do better than those who don’t. This is certainly the case during the launch phase of a new medicine, when both real-time and longitudinal analysis of formulary trends can strengthen decision-making and help accelerate commercial success.
Research consistently shows that – good or bad – the first six months of a product’s life significantly shape its long-term commercial success. McKinsey argues that 65% of launches that exceed sales forecast in year one continue to flourish in their second year, while 75% of those that fall short of Y1 projections continue the same trajectory the following year. While these figures double down on the need for launch excellence, pharma’s recent track record at launch is underwhelming.
Around two-thirds of new drugs fail to meet pre-launch expectations, while in the past decade, the average ROI in pharma R&D has slowly declined. Better visibility – and interpretation – of formulary activity can play an important role in reversing the trend and accelerating growth at launch.
Analysis indicates that formulary intelligence can be a major differentiator, allowing companies to maximise the headline characteristics of a brand’s value proposition that, without effective targeting, may not have the predicted impact at launch.
So, if you’re launching a new product, what percentage of formularies can you expect to get in year one (Y1)? This is a common question. The answer naturally depends on a brand’s value proposition to prescribers and payers. Nonetheless, irrespective of the offering, research suggests that the industry’s Y1 expectations (and projections) are markedly different from the reality.
Five years ago, we tracked the Y1 formulary adoption of five high-profile UK product launches in respiratory and diabetes. Each brand had value propositions, promotional heritage and support that differed – but to the outside eye, each could have reasonable expectations of a healthy Y1 uptake:
- Abasaglar – a biosimilar to Lantus (diabetes), offering a money-saving value proposition for medicines management
- Sirdupla – a branded generic to Seretide (asthma), offering good cost savings for medicines management
- Trulicity – an NCE (T2 diabetes), the first readyto-use once weekly GLP-1 receptor agonist to reach the market
- Spiolto – a LAMA/LABA combination for COPD and heir to Boehringer’s blockbuster Spiriva
- Toujeo – a long-acting insulin injection (diabetes) and heir to Sanofi’s #1 insulin brand Lantus.
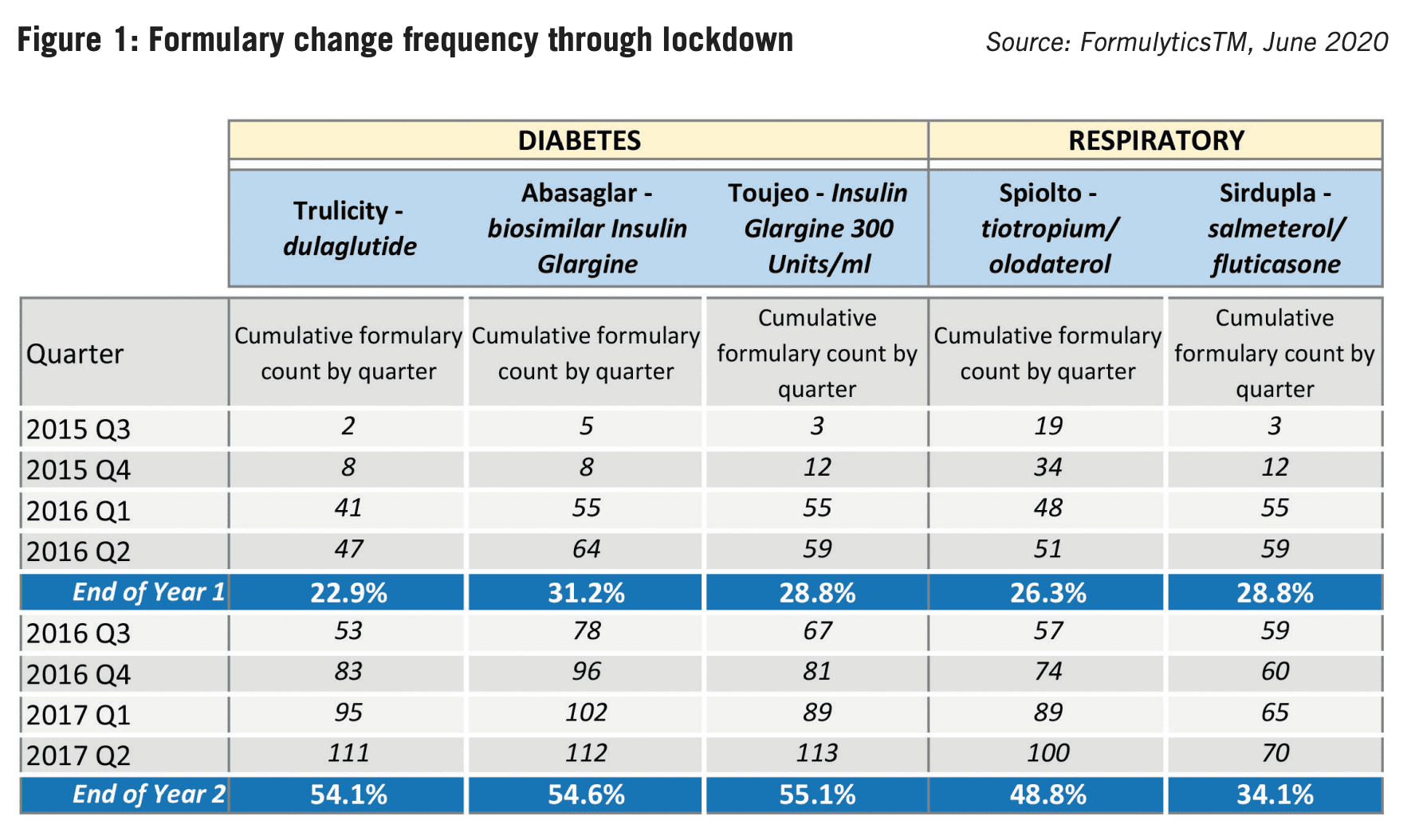
This sample encompassed a range of product profiles – from money-saving biosimilars and branded generics to novel therapeutic innovation, priced accordingly. It also included manufacturers with significant bulk in promotional resources and vast experience in their therapeutic fields.
At the time, polling of a sample of companies anticipated Y1 formulary adoption ranging between 50-80%. The real-world results were surprising (see Figure 2). The best uptake at the end of the first twelve months was 31.2% of all formularies. Mean uptake was just 27.6%. The lesson? It doesn’t matter what your product profile is, the NHS is slow to adopt. Moreover, the onus is on pharma companies to target resources effectively to secure pull-through. There isn’t a magic wand.
Being passive and expecting your value proposition to do the talking will only result in disappointment. Formulary inclusion isn’t a guarantee of product sales, but it’s a vital first step on the ladder of adoption. If you fail to understand – and plan for – the complexity and diversity of the formulary landscape, you will inevitably fall short of commercial expectations in the crucial first year of launch.
However, with good visibility of the formulary environment – and good analytics to help you interpret and respond to formulary intent – you can have great success. For example, robust and agile real-time formulary intelligence recently helped a leading diabetes player achieve Y1 formulary uptake of 59% with a launch product – more than double the mean percentage from our 2015/16 sample. The question is: how?
A winning formula
Good formulary intelligence is much more than a static repository of documents or automated notifications of change – it’s a composite of realtime information, longitudinal analysis and expert interpretation. Formularies are dynamic, diverse and locally-nuanced, and they do not conform to a standardised playbook. Understanding – and planning around – them requires in-depth knowledge of complex market access environments. If you’ve got all that, the potential to accelerate commercial growth is real.
Robust and timely insight can power predictive modelling to support strategic planning and forecasting. It can help you prioritise accounts and activity not just according to sales potential and basic demographics. It can provide analogues that identify early formulary and prescribing adopters – so you can target access activity to early formulary adopters, and target sales activity and digital campaigns to early prescribing adopters who prescribe pre-formulary.
And it can help you de-prioritise laggard formularies whose sphere of influence is limited. Agile formulary intelligence should be a key component of launch excellence, but in too many pharma companies it’s undervalued and overlooked.
As the industry battles to arrest the decline in R&D ROI and accelerate the commercial value of new innovation, the most successful marketers will be those that recognise the value of formulary information and use it to inform access strategies, sales forecasting, customer targeting and differential detailing.
In the process, they’ll free access teams to focus on customer engagement – and empower them with vital intelligence that enhances the interaction and drives brand adoption. If what gets measured, gets done, just imagine what’s possible when you can measure things properly.




