Cancer treatment has been transformed in the last few years, thanks to that now-familiar generation of drugs which strip tumours of their ‘cloaking device’, allowing the body’s own T-cells to identify and fight cancer: the PD-1 and PD-L1 checkpoint inhibitor immunotherapies.
BMS led the charge when Opdivo became the first in its class to gain approval (in metastatic melanoma in December 2014 in Japan), but Merck & Co’s Keytruda has emerged as the outright winner in the category. It sealed its dominance last year with a string of successful endpoint-hitting trials, most especially in non- small cell lung cancer (NSCLC).
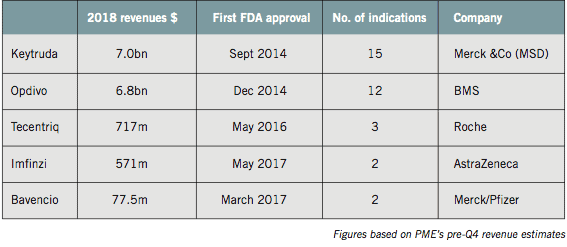
Keytruda is expected to achieve full year 2018 sales of around $7bn, nearly doubling its sales revenues over the course of 12 months. Opdivo will be just behind on around $6.8bn, with more rapid growth for both therapies expected this year.
Now approved in no fewer than 15 and 12 different indications respectively, Keytruda and Opdivo are leading the checkpoint inhibitor push into new tumour types, earlier disease settings and new combinations.
In addition to the PD-1 inhibitors Keytruda and Opdivo, there are four PD-L1 inhibitors: Roche’s Tecentriq, AstraZeneca’s Imfinzi, Pfizer and Merck’s Bavencio and Sanofi and Regeneron’s newcomer, Libtayo.
However this class of drugs aren’t miracle cures: many patients and tumour types do not respond to PD-1/PD-L1s – the overall response rate (ORR) is commonly well below 50%. The true figure for patients treated with checkpoint inhibitor monotherapy achieving durable and meaningful clinical responses is in fact nearer 20%.
And while progress in extending the lives of patients has been impressive across many tumour types, most overall survival (OS) gains over existing treatment are still measured in months rather than years.
All this leaves plenty of room for improvement. That’s why the sector has initiated hundreds of combination trials in the last few years, looking to twin these drugs with new or existing therapies that can boost ORR and OS.
PD-1/PD-L1 inhibitors: Keytruda is King, but the market is developing fast
PD-1/PD-L1s as a whole will remain the industry’s most significant growth drivers in 2019, and more new arrivals are expected to join the crowded field over the next few years.
Analysts at Research and Markets said the global immune checkpoint inhibitors market was valued at $10.5bn in 2017, and is expected to reach $65.4bn by 2025, growing at a CAGR of 25.6% from 2018 to 2025. Of this total, treatments for lung cancer represented nearly half of the global market share in 2017, and are expected to continue rapid growth in 2019 and beyond.
Here are some highlights from how the sector progressed in 2018, with a look ahead at some of the key developments in 2019.
Keytruda extends its lead

Keytruda established itself as the new standard for first-line treatment of metastatic non-small cell lung cancer (NSCLC) – and also finally overtook its main rival, BMS’ Opdivo in revenues in Q2 of last year.
This success rests on numerous trials, but its biggest bullseyes last year were as a first-line combination with standard chemotherapy regimens in non-squamous NSCLC (KEYNOTE-189, which confirmed its efficacy after conditional approval via another study) and in squamous (KEYNOTE-407) NSCLC as compared to these existing regimens alone.
These indications have now both been approved in the US (with Europe expected to clear the squamous use soon) and are set to be key drivers for Keytruda’s growth in 2019.
The only drug that has any realistic chance of challenging Keytruda in NSCLC is Roche’s Tecentriq. The company is exploring its use in a variety of double and triple combinations.
In December the FDA approved its use in combination with Avastin and chemotherapy in non-squamous NSCLC patients, based on results from the IMpower150 study.
An FDA ruling on Tecentriq in combination with carboplatin and nab-paclitaxel (Abraxane) as a first-line treatment for patients with metastatic non-squamous NSCLC (based on the IMpower130 trial) is expected by 2 September.
The Tecentriq + chemo trial, IMpower132, is the most important head-to-head with Keytruda (in non-squamous NSCLC). However the last data read-out showed this hit its progression-free survival (PFS) endpoint but not OS – handing a clear advantage to Merck & Co.
Frontline monotherapy in NSCLC
The next regulatory milestone for Keytruda will be FDA approval as a first-line monotherapy in locally advanced or metastatic non-squamous or squamous non-small cell lung cancer NSCLC patients with low PD-L1 expression (≥1%, also without EGFR or ALK mutations) – a group that was once thought unlikely to benefit from these drugs.
This is a very significant segment of patients – as many as 40% of first-line patients fall into this category.
This will be based on the KEYNOTE-042 trial, which was presented at ASCO last year, where it looked like another hands-down victory for Merck & Co.
Keytruda monotherapy showed significant improvement in overall survival compared with chemotherapy in this patient population – 16.7 months versus 12.1 months. It creates a new dilemma for doctors talking to patients about whether they should be given Keytruda as a monotherapy or in combination with chemo, which many patients would prefer to avoid.
It’s not entirely clear yet whether chemotherapy could eventually be dispensed with in first-line NSCLC, though KEYNOTE-042’s results show slightly superior OS in cross comparisons with the Keynote combination trials.
The FDA had been expected to grant approval in January, but Merck & Co submitted substantial extra data, resulting in a new Prescription Drug User Fee Act (PDUFA) decision day of 11 April. Naturally Merck & Co isn’t confining itself to dominance in lung cancer.
On 28 December, Keytruda gained FDA clearance for use in Merkel cell carcinoma. This is particularly bad news for Pfizer and Merck’s Bavencio, as it has had this indication to itself until now, and has suffered several failures in attempting to broaden its use into other tumour types. Bavencio has its own shot at being a first-line NSCLC monotherapy in its Javelin Lung 100 study, with a read-out expected in October 2019, although this looks unlikely to produce encouraging results.
For Merck & Co, the success of Keytruda means it has committed itself to enormous investment in expanding its huge clinical trial programme for the drug, as well as major investment in its marketing and sales efforts to maximise its market share.
“This is a period where we have to invest because there’s never been this kind of opportunity for us,” Merck & Co’s CEO Kenneth Frasier told analysts at the JP Morgan conference in January.
He added: “It’s unprecedented in terms of the scope. Keytruda is approved today in ten tumour types, including lung. We’ve shown activity in 25 tumour types. So there’s a huge amount of investment that’s necessary both in monotherapy as well as in combinations across all the different lines of therapy.” BMS bets on novel combinations Despite being eclipsed by Keytruda in NSCLC and overall revenues, Opdivo is still very much ahead of the other products in the class. Opdivo is not a contender as a monotherapy or combination with chemo in front-line NSCLC, having failed key trials in the last few years. However, the company continues to study Opdivo in trials twinned with a very wide range of novel agents, with the hope of finding a combination which can take immuno-oncology (IO) to the next level.




