
AstraZeneca’s head of Europe and Canada Iskra Reic
Iskra Reic was appointed executive vice president of Europe for AstraZeneca in April 2017 and in a reshuffle at the beginning of 2019, Canada was added to that portfolio.
However, it was in another huge and snowy country where she really cut her management teeth – Russia.
Trained as a doctor of dental surgery at the Medical University of Zagreb in her native Croatia, Reic joined AstraZeneca in 2001 and quickly ascended through the company, heading up Specialty Care in Central and Eastern Europe, Middle East and Africa before landing the general manager role in Russia in 2014.
Under her leadership, AstraZeneca achieved a leading share in its three main therapy areas and became a top three prescription medicine pharma company in Russia.
Reic’s responsibilities were expanded in 2016 to cover both Russia and the Eurasia Area, where she led a 1,500-strong team in an ‘emerging market’ region. Such regions can provide rapid growth, but can also prove to be frequently volatile and unpredictable.
Reic said her time in Russia in particular has taught her the power of tenacity, flexibility and a can-do spirit – not just as a leader, but for the whole team working in a market that can change overnight.
“Russia is an exciting market to work in, but you have to be really committed to it to get through the bad days.
“We brought a lot of innovative new medicines to patients there, which involved intensive work in educating healthcare professionals and the government about the value of innovation in pharma.”
During her time there the rouble was hit by a major devaluation.
“That has a big impact on any business. In those changeable markets, you really need to build a long-term model which can adapt to those sudden developments.”
Reic’s move to taking on the whole of Europe came two and a half years ago, and coincided with a renaissance in AstraZeneca’s fortunes, which have been in the doldrums due to the expiry of old blockbusters such as Nexium and Crestor.
Now the business has two particularly strong growth drivers: emerging markets, most especially China, and oncology, where a triumvirate of drugs – Tagrisso, Imfinzi and Lynparza – have hit a major growth phase.
So strong has Chinese growth been for the company that it has already overtaken Europe as the second most important market for AstraZeneca.
That does beg the question of how the company will assign its budgets in the long term, especially as Europe is still lagging behind the US and China in its return to growth.
Europe
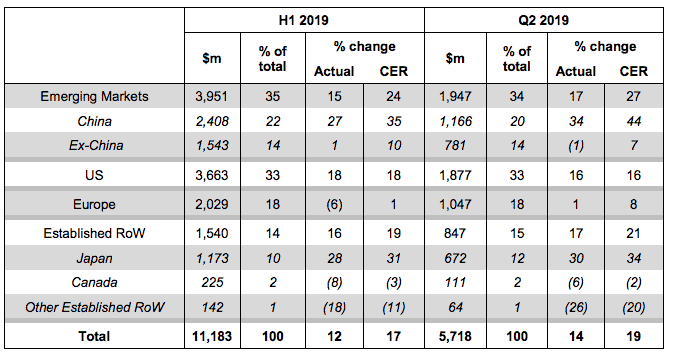
Reic said: “At AstraZeneca, Europe is tending to run one year behind markets like the US, but I’m very encouraged by Europe’s return to sales growth in the second quarter of 2019, increasing by 8% (CER) to $1.047bn.”
She noted that Europe represents around 20% of the business and is an important region now and for the future.
“Beyond the size of the market, a strong presence in Europe also gives you excellence in payer engagement, and a greater sophistication in market access and building innovative value strategies.
“Finally, I think we all recognise that Europe is an important talent pool for any global pharma organisation, which can be a source of great competitive advantage.”
She concluded: “Altogether, I think those three elements – the market size, its lead in payer engagement approaches and its talent – will all keep Europe at the forefront.”
European policy matters
Another aspect of any pharma leader’s job in the region is Europe’s environment, including the EU’s policy direction. Naturally, Brexit is one short- to-medium-term threat, but the industry is even more focused on where the EU is heading in terms of the region’s attractiveness to inward investment and the ease of market access.
Reic is in step with leaders at EFPIA in saying the EU needs to prioritise investment in science and R&D to maintain the region’s pre-eminence for life sciences.
“This should be top of the agenda, including proposals to streamline health technology assessment (HTA) across the EU,” said Reic.
The proposals centre on creating a centralised procedure for the clinical assessment of new medicines, which would eliminate the cost and time spent on duplicating this process with regulators and HTA agencies.
However, some member states remain firmly opposed to creating a mandatory centralised system, worried it could undermine the independence of their healthcare decision-making.
“This proposal has to make this centralised procedure mandatory. The reason is very simple – making its adoption optional will have the opposite effect and will slow down patient access to innovative medicines. That’s because optional uptake would only serve to add an additional regulatory layer, rather than provide any benefits to patients.”
Making the most of your talent
Returning to its talent pool, one of those three key strengths of Europe, something else that is also naturally high on Reic’s list of priorities is making the most of the company’s human resources.
She said finding and developing the right blend of cross-functional skills and capabilities is now more vital than ever. New emphasis is now being put on diversity in teams, including efforts to ensure women with leadership potential are identified and nurtured.
“Our CEO Pascal Soriot chairs quarterly meetings of our Global Inclusion and Diversity council, the group that sets our Global standards and steers implementation on inclusion and diversity. Inclusion is the foundation of our ability to innovate and make the most of our diversity of thought.”
So what will it take for the next generation of women to follow in her footsteps and take their fair share of the top roles?
“I think it’s about confidently expressing your ability – or perhaps more importantly, just your willingness to do something, versus being afraid of failure.
“That’s something women could be better at. It’s certainly the most frequent topic that comes up when I’m mentoring women for leadership roles.”
Europe’s market access and competitiveness
Of course, that highly developed payer environment also helps to put the brakes on uptake of new medicines in Europe.
One development which could address this problem is the move toward value-based agreements. Healthcare systems in the region are increasingly asking for more robust evidence of impact on patient outcomes from new medicines.
“Europe is complex when it comes to access and reimbursement. How we work with payers now and in the future will be a critical factor in how we bring innovative medicines to patients. That means we need to have a discussion around value, and actually show the value our medicines bring to patients.”
Like many of its peers, AstraZeneca now firmly believes in what Reic calls “strictly linking reimbursement to the value and the clinical outcome” of a medicine.
So does AstraZeneca believe there might be one value-based model that could be applied across global markets?
“No, I don’t think there will be a single model for the US and Europe, and certainly no single model for Europe either, one which you could use with NICE in England and with the payers in Poland as well, for example.”
Despite the complexity of this puzzle, it is one that has to be cracked.
“For more than a year we have been developing a range of models that will address the different criteria that payers have. Having said that, in essence all healthcare systems want the same thing: data that proves your product produces the desired clinical outcomes – not only from clinical trial data, but also in a real-world setting.”
She added: “Being able to show that real-world benefit, in a specific population in a specific market, makes a huge difference in those negotiations.”
An example is the landmark CVD-REAL study of more than 300,000 patients. This is the first large real-world evidence study of its kind, evaluating the risk of hospitalisation for heart failure and death from any cause in patients with type 2 diabetes receiving treatment with SGLT-2 inhibitors, including AstraZeneca’s Forxiga (dapagliflozin).
Diagnostics and cracking the market access code
For many of the company’s new generation of treatments, the uptake of companion diagnostics into routine clinical practice is another part of the market jigsaw which has to fall into place.
Two leading examples of this are Tagrisso, which is now the leader in EGFR+ lung cancer treatment, and Lynparza, which targets BRCA and other mutations in multiple tumour types and is the only PARP inhibitor with positive phase 3 results in four different cancer types (ovarian, breast, pancreatic and prostate).
Reic said the adoption of diagnostics into standard clinical practice has been one of the biggest challenges in Lynparza’s first indication, ovarian cancer – but this must now be replicated in all the other tumour types in which it has already been approved or is pending approval.
The drug has so far gained approval for use in patients with germline BRCA1/2-mutated (gBRCAm), human epidermal growth factor receptor 2 (HER2)- negative locally advanced or metastatic breast cancer, previously treated with chemotherapy.
Approvals in BRCA-mutated pancreatic and prostate cancer are also expected in the near future, two tumour types where currently physicians aren’t accustomed to using these tests.
The company believes that its recent phase 3 read-outs (POLO in pancreatic, PROfound in prostate and PAOLA-1 in advanced ovarian) demonstrate the potential value of genomic testing in at-risk patient populations.
“This is the kind of challenge you come up against when you have an incredibly exciting molecule that is generating lots of data,” she said.
“First we had ovarian cancer, then breast cancer, and our next steps will be for metastatic pancreatic cancer and men with metastatic castration-resistant prostate cancer. Those all bring unique challenges, but we’re building experience and expertise as we go.”
Looking to build its momentum in oncology even further, AstraZeneca recently signed a huge $6.9bn deal with Daiichi-Sankyo to develop and co-market its next-generation challenger to Roche’s Herceptin.
That’s a measure of its heightened ambitions in oncology, especially as Roche is dominant in the HER2 breast cancer field.
Reic concluded: “It’s an opportunity to transform treatment of breast cancer. We believe this antibody drug conjugate has the potential to redefine the treatment of patients with HER2-expressing cancers, and which could become the first therapy for HER-2 low tumours. It really fits with our ambition and our focus, which is all about enriching our pipeline and advancing the science for the benefit of patients.”




