
Regulators around the world have been working for decades to speed up access to the best of the new medicines being developed and brought to market. The European Medicines Agency (EMA) is no different offering five routes that can hasten approval, under certain conditions. The most recently added – the PRIority Medicines (PRIME) scheme – reached its two-year anniversary in March 2018.
So how has it gone? Leela Barham looks not just at the EMAs report of the first two years but what it might mean for patient access beyond regulatory approval.
The scheme
The PRIME scheme is for medicines that have real promise, defined by the EMA as medicines that may offer a major therapeutic advantage over existing treatments, or benefit patients without treatment options. Companies who want to benefit from the scheme have to convince the EMA – based on early clinical data – that their medicine has the potential to benefit patients with unmet clinical needs.
Getting into the PRIME scheme offers a number of benefits. They include early appointment of a rapporteur to provide support and guidance for later application for marketing authorisation, a kick-off meeting with the rapporteur and experts, a single contact point throughout PRIME, scientific advice and confirmation of the potential for accelerated assessment (another way to speed things up) at the time that an application for marketing authorisation is put in. Going for scientific advice once given the PRIME designation can also mean faster advice: a 40-day timeframe versus the 70-day standard. SMEs can also get a filing fee reduction.
PRIME numbers after 2 years
· 177 requests for eligibility, or 8 a month on average
· 71 requests have been chemical products, 43 biological, 42 ATMPs with the last 13 classed as ‘other’
· 72 requests have been underpinned by Phase 2 data, 43 Phase 1-2, 35 Phase 1 and the remaining 11 a mix of Phase 3, compassionate use/case series data or the literature
· 8 requests have been out-of-scope
· 36 requests have been granted eligibility for PRIME, or around a 20 percent acceptance rate across all therapy areas
· 30 PRIME treatments are in rare diseases, and16 for paediatric patients
· 31 kick-off meetings
· 37 scientific advice sessions for 22 treatments
Making the PRIME grade
Just over half of requests that were granted for PRIME by March 2018 are for treatments in oncology and haematology-haemostaseology (figure 1). This reflects some big differences in acceptance rates across therapy areas (figure 2).
Figure 1: Treatments with PRIME status by therapeutic area
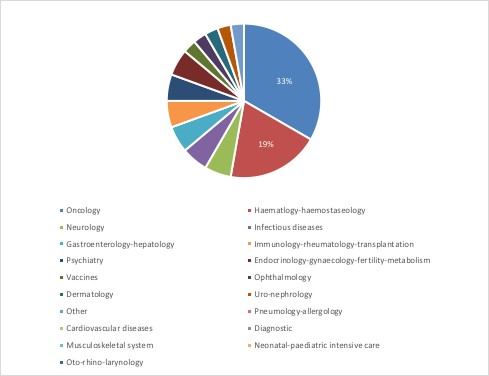
Source: Analysis using EMA data after two years of PRIME. n = 36
Figure 2: Acceptance rates by therapeutic area
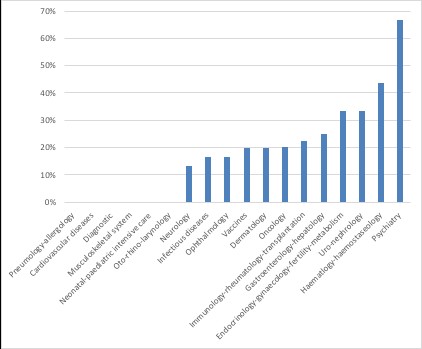
Source: Analysis using EMA data. n = 36
PRIME isn’t just for big companies: up to March 2018 just nine of the companies – with 10 PRIME treatments – are in the top 50 companies of 2017 by sales, and 6 are in the top 10 companies with Pfizer (with Spark Therapeutics), Novartis (by acquiring AveXis), Celgene (with Bluebird and through acquiring Juno Therapeutics), Sanofi, Roche and Merck all being part of the scheme.
Since March 2018 Roche, with Ionis, have added another PRIME drug to their portfolio. RG6042 (formerly named IONIS-HTTRx) is in development for Huntington’s disease. PRIME has come as a result of promising results in a phase I/IIa study which shows the ability of RG6042 to reduce the toxic mutant huntingtin protein (mHTT). It’s hoped it could be a big step in treatment for the genetically-inherited and rare wasting disease. PRIME status is being touted as useful to help advance development of RG6042.
Still work to do
The EMA has proactively sought feedback from those who have participated in PRIME kick off meetings to find out how well they’re doing. On the whole it seems most are happy (figure 3); there is still work to do particularly to improve on how the EMA follows up.
Figure 3: Agreement with statements on PRIME

Source: Data from EMA. n = 15.
Too soon to tell if PRIME time will mean payment
EMA points out that there are three treatments in the PRIME scheme that were undergoing marketing authorisation assessment at the two-year point. Early signs are looking good: Novartis’ Kymriah (tisagenlecleucel) and Gilead/Kite’s Yescarta (axicabtagene ciloleucel) secured positive opinions from EMA’s Committee for Medicinal Products for Human Use (CHMP) in June 2018. However getting that seal of approval from the regulator is but one stage on the pathway to getting a treatment to patients and, crucially, paid for.
It’s too soon to know if those treatments getting the PRIME treatment will go on to be given approval by payers – including Health Technology Assessment (HTA) agencies – or not. There could be some grounds for optimism though: the PRIME scheme includes the option for HTA agencies – and patients – to be involved in giving scientific advice. So far, 37 sessions of scientific advice have taken place for 22 products, with seven including HTA agencies and six patients.
That could help to overcome issues with the evidence that payers want to see.
What may prove to be a stumbling block for patients getting access – and companies getting revenue – could well be cost. Kymriah and Yescarta might well enter the European market with a list price of EUR300,000. Those prices pale in comparison though when compared to the speculation that the price tag for Spark Therapeutics and Pfizer’s PF-06838435/SPK-900 could come in somewhere in the region of $1million to $2 million per patient.
No amount of emphasising the promise of the value of the drug and early engagement with HTA and payers will ease the sticker shock of those kinds of prices. That may mean that getting marketing authorisation, even if it happens more quickly, won’t necessarily translate into speedy access to reimbursement. The end result could well be a stand-off between payers and companies on price.





