Clarivate Analytics, through its Centre for Medicines Research (CMR) International group, recently released the 2019 CMR International Pharmaceutical R&D Factbook –providing insights into drug development costs and approval trends based on data and analyses from multiple proprietary data sets. This report highlights the changing nature of drug approvals, setting the stage for what we might expect in the future.
Overall, in 2018, new molecular entity (NME) output grew once again with a corresponding decrease in the cost per NME. Based on the data, this trend can be attributed to an increase in activity from smaller pharmaceutical companies as well as an increased focus on rare diseases. Deal- making has impacted R&D and when considered together with drug pricing pressures, these trends raise the question of pharmaceutical company sustainability.
Approvals by company size
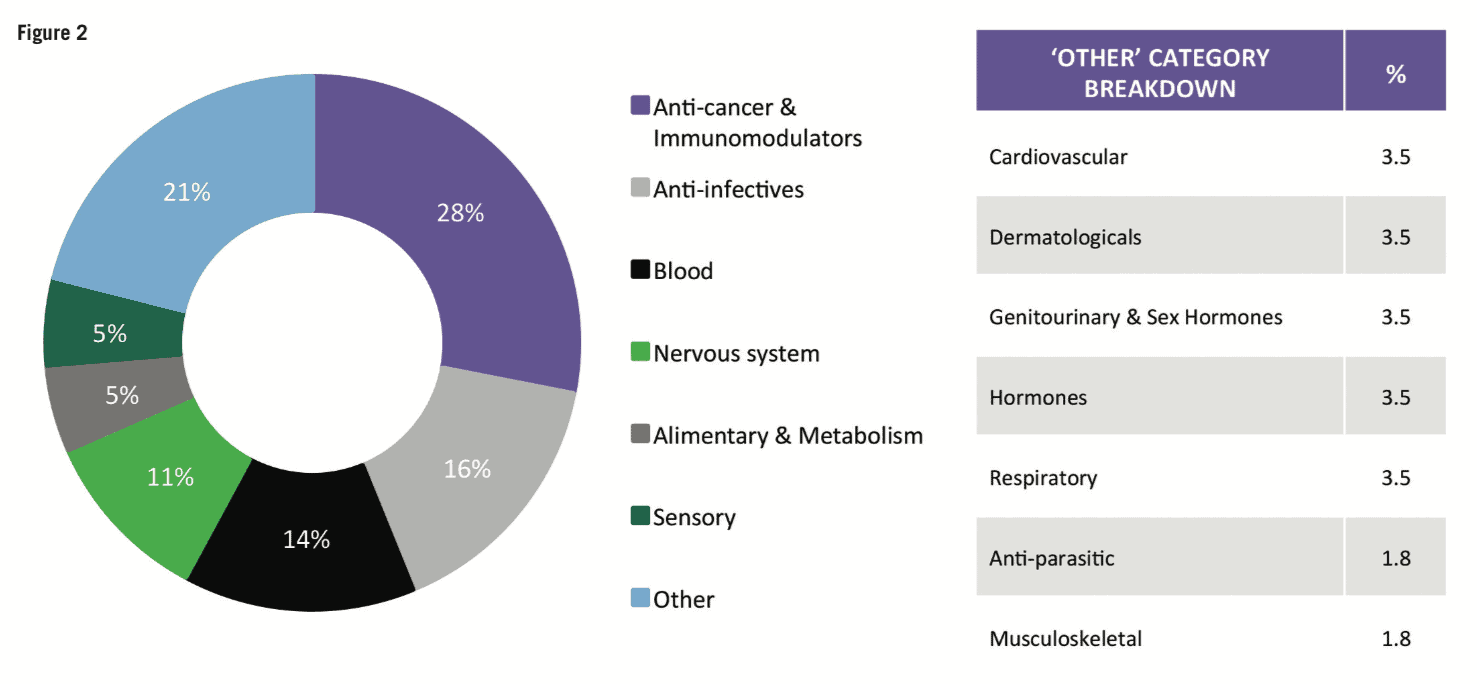
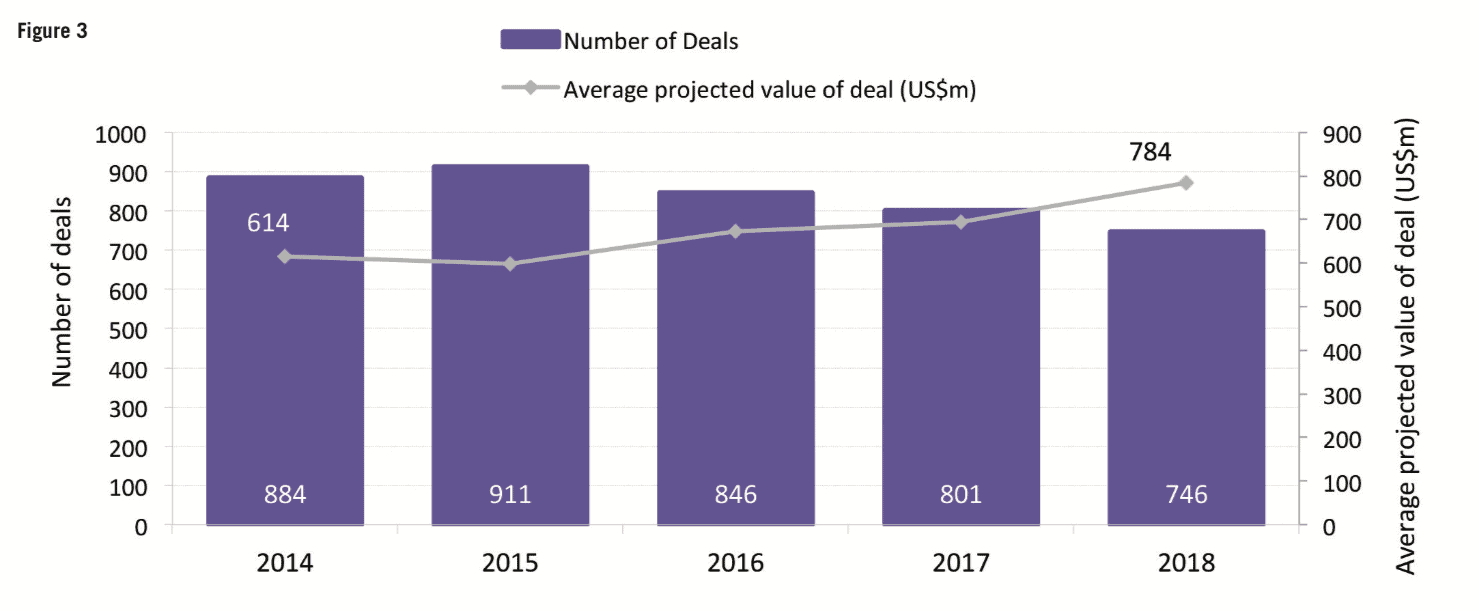
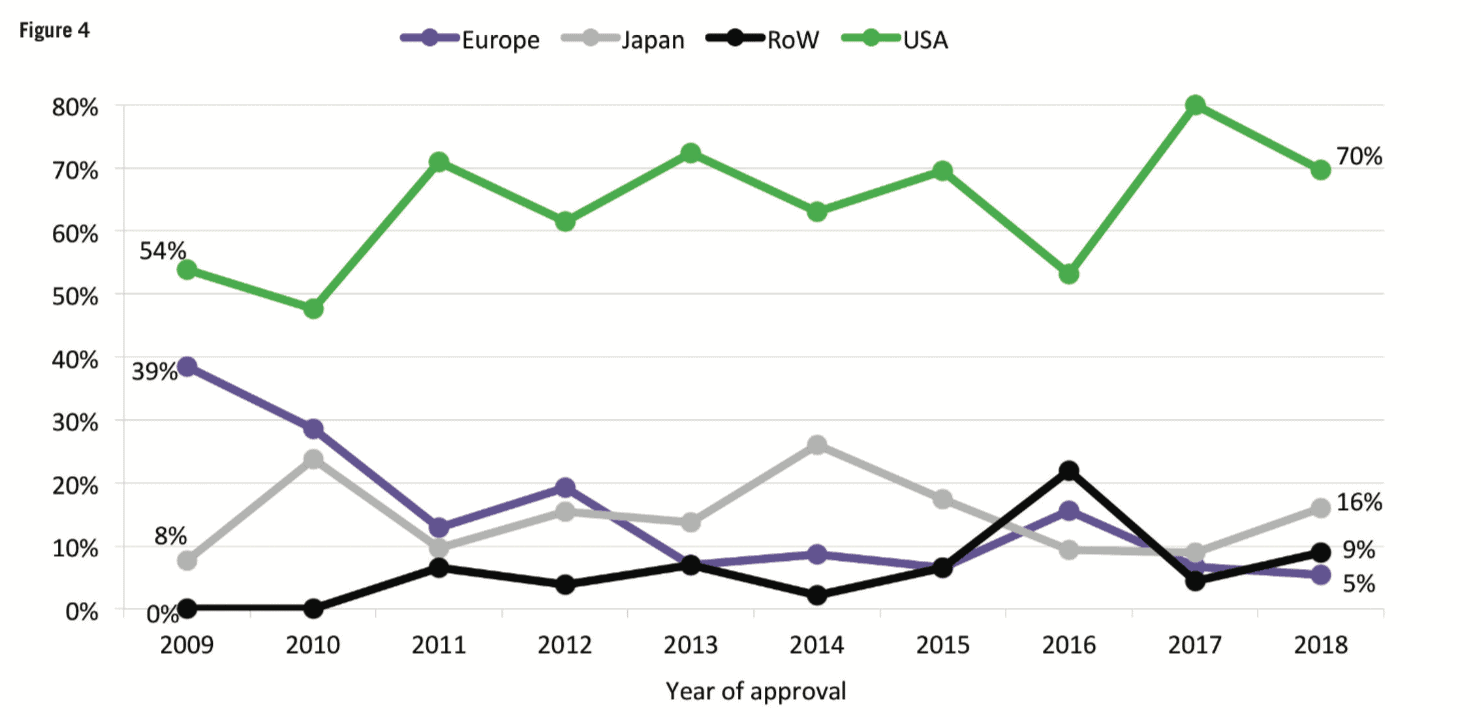
For the first time in a decade, mid-sized and smaller companies (those with R&D spend of Small and mid-sized companies can concentrate on specific development areas, innovative products and diseases with smaller target populations. Compared with larger companies, they tend to have a narrower business focus and be more agile, with smaller overheads. Shift in therapeutic areas Oncology drugs continue to be a core part of companies’ development strategies. At 29.5% of total R&D spend and 28% of first NME launches, oncology represented the largest proportions for both metrics in 2018. However, rare diseases and orphan drugs are also emerging as important focal areas. According to CMR First World launch data, of the 57 NMEs launched in 2018, 22 had an orphan drug designation. The incentives (market exclusivity, reduced regulatory fees, subsidies) introduced for orphan drugs under the US Orphan Drug Act (ODA) and later with the Committee for Orphan Medicinal Products (COMP) in the European Union have encouraged companies to develop drugs that would otherwise be considered poor investments. In fact, the FDA reports that more than 450 products have been approved since the ODA, compared with only ten drugs prior to that time. And, except for oncology, pipeline volumes decreased across all major therapeutic areas between 2016 to 2018, with the most notable reductions in respiratory, musculoskeletal and central nervous system. Of particular note is the decline in central nervous system development – of the seven analysed therapeutic areas, central nervous system active substances have the lowest probability of success to market across the phases of development represented in the analysis and, until the recent about-turn by Biogen, the outlook for neuroscience was bleak. High-profile disinvestments include those by Pfizer and Eli Lilly. However, Pfizer has since announced that, together with investment firm Bain Capital, it has created a new drug company called Cerevel Therapeutics to focus on therapies for Parkinson’s, epilepsy and other central nervous system disorders. Increased deal-making Increases in deal-making have changed the R&D landscape. In 2018, about half of all approvals were initiated through pipelines that were sourced from outside and/or through innovation that was initiated by other companies. It is safe to say that the days when companies would bring products from the lab through the clinic to approval are long gone. Instead, many companies are looking to refocus their therapeutic area coverage, often picking up assets developed elsewhere. Mega-mergers are also becoming less frequent; instead, we’re seeing fewer but larger deals. Based on CMR International data, since 2015 we have seen the number of drug commercialisation/licensing deals continue to gradually decline while the projected deal value rose by almost 31% during the same time frame. Not everything changes Still, as much as things are changing, some things stay the same. The US continues to be the dominant region for approvals – 70% of new drugs were filed first in the US. Among other factors, the US reimbursement policies remain attractive for companies. In contrast, less than 20% of new drugs were filed first in each of the other regions (Europe, Japan, RoW). Industry sustainability These trends feed the debate around the sustainability of the pharma industry. Even as we note that 2018 was a banner year for approvals, we aren’t seeing significant declines in R&D costs or cycle times. In fact, although we saw a significant decrease in clinical development times for NMEs first launched on the world market from 2010 to 2013, they have since increased by approximately 7%. And, according to our analysis, estimated sales per approval are declining. The average peak sale amount per asset in 2015 was more than double that in 2018. With the trends for greater approvals in the US, drugs developed by smaller companies and drugs targeting smaller patient populations, the question of sustainability becomes all the more relevant. Vision for the future As we look forward, sustainability will continue to influence company strategies, particularly given that some new drugs, such as Zolgensma for spinal muscular atrophy (SMA), cost US$2m or more per patient. At the same time, payers will likely implement more rationing and/or price controls. We will continue to see geographic and therapeutic rebalancing. Growth in sales volume is slowing in the US and Europe, while greater activity has been observed in Mainland China. Regulatory processes in Mainland China have become more streamlined, resulting in greater investment by global pharma companies and more approvals of innovative medicines. Therapeutically, oncology and rare diseases will likely remain attractive candidates for investment, while market failures for neuroscience and anti-infective therapies will continue to negatively affect investment in those areas. Digital technologies and artificial intelligence are creating new opportunities to identify new targets, leverage real-world data, rapidly test hypotheses and support clinical decision-making. Along with this, however, comes new challenges such as integrating the data sources needed to support robust machine learning. Therefore, the industry needs to develop solutions that provide a single source of truth to inform the decision-making process. Finally, 2019 is tracking to be on par with 2012 and 2017 for the highest number of CEO changes within pharma companies and has the second highest number of changes within the The analysis from CMR International highlights important questions about the sustainability of the pharmaceutical industry and provides objective insights that can inform discussions about how to move forward. It will be important to use ever-more sophisticated data sets to continue to understand pharma’s ongoing evolution.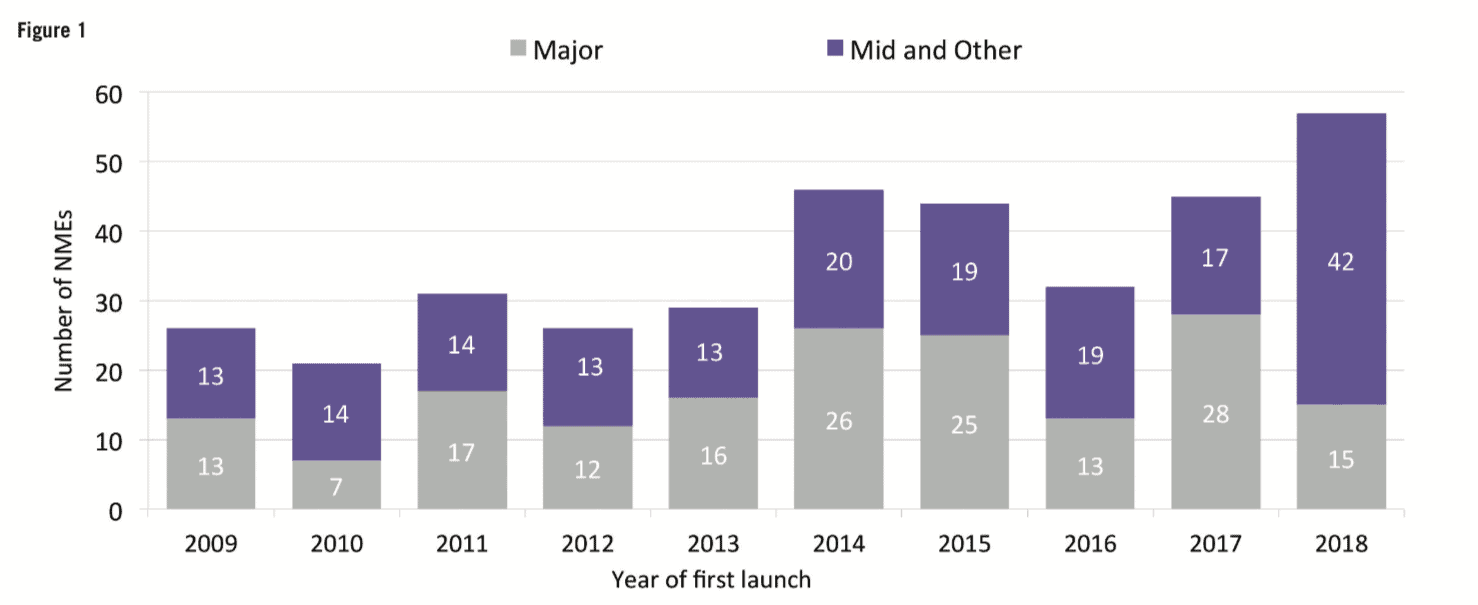
top 12 pharma companies. With these significant leadership changes, we should expect to continue to see additional changes within the industry for at least the next few years.





