
Although the economic burden imposed by rare cancers has not yet been adequately assessed, the treatment burden posed by rare cancers on individuals, societies and healthcare systems make them a public health priority. With the increasing burden of rare cancers, there is a high demand for early access to orphan drugs from all quarters. In order to facilitate better access to orphan drugs, governments have devised special access programmes. This article discusses one such early access programme called ‘Compassionate use programme’ in the EU28 which is in operation through set regulations and guidelines at European and national levels.
Burden of rare cancers
Rare cancers affect over 4.3 million people in the European Union (EU), with more than 540,000 new cases diagnosed annually – that is roughly the population of Luxembourg. There are over 198 rare cancers identified globally, which collectively embody about 22% of all the cancer cases in the EU 28 member states (EU28) each year. The usage of ‘rare’ is an anomaly considering the epidemiological profile of rare cancers. Rare cancers severely affect patients’ quality of life, with the five-year relative survival rates being lower for rare cancers than for common cancers. Rare cancers require a multimodal treatment approach using surgery, radiotherapy and anti-tumour agents. This approach entails multidisciplinary teams, including surgeons, radiotherapists, medical oncologists, diagnostic radiologists, pathologists, specialised nurses and psychosocial support. Thus, the health burden and economic encumbrance posed by rare cancers on individuals, societies and health systems are enormous owing to the direct and indirect costs of care. Remarkably, the cost of drugs constitutes a major chunk of the total direct costs of rare cancers, especially for the innovative drugs also termed orphan drugs.
Challenges of early access to orphan drugs
In Europe, orphan drugs are used to treat life-threatening or chronically debilitating diseases, usually with the prevalence of not more than 5 in 10,000 (including rare cancers). At times, these innovative drugs are comparatively more expensive than conventional cancer drugs. Given this, it is becoming increasingly difficult for governments and payers to include them in their reimbursed drug lists, causing disparity in access to novel drugs. Another challenge in the patient access to orphan drugs in the EU is the discrepancy in the regulatory scenarios across different countries for cancer drugs.
The marketing authorisation for novel therapies is often time-consuming, causing distress to some patients, particularly those with rare cancers. Sometimes, new drugs that are unauthorised or in the late phase of clinical trials may be the last resort for such patients. Previously, clinical trials were the only way for patients in many countries to access drugs under development. However, participation in clinical trials is not easy for rare cancer patients due to elaborate inclusion and exclusion criteria, time and physical condition, etc. On the whole, rare cancer patients face specific challenges due to the lack of access to appropriate therapies and clinical expertise, and limitations that hinder pharmaceutical companies from entering a market. So, there is a necessity for programmes which promise a simplified or accelerated approval procedure for orphan drugs.
Rare cancers affect over 4.3 million people in the EU, with more than 540,000 new cases diagnosed annually
Compassionate use programme
In recent times, early access programmes have opened up more possibilities for rare cancer patients. The Compassionate Use Programme (CUP) is one that has achieved profound success. Since 2010, compassionate use requests have risen by nearly 25% and a major proportion are from the rare disease patient populations (including rare cancers). Governments and pharmaceutical companies are increasing their efforts to improve early access to drugs for life-threatening diseases. So, it is essential to understand CUP and the processes involved.
CUP is an early access programme that makes new medicines available to patients with life-threatening disorders or diseases in the EU28. The European Medicines Agency (EMA) defines ‘compassionate use’ as a treatment option that allows the use of an unauthorised medicinal product under development. In general, the use of CUP is considered in the early stages of product development where patients get pre-launch access to the investigational or unauthorised drugs that are available in their country. Unlike protocol-driven clinical trials, where participants need to meet certain inclusion and exclusion criteria, CUP does not consider any criteria, but there are laws and regulations to consider when using CUP.
The EMA recommends compassionate use through the Committee for Medicinal Products for Human Use (CHMP) and sets the laws and regulations for its use in the EU. Every EU member state has its own legislation for CUP based on the EMA recommendations and legal framework. Therefore, it is necessary for stakeholders like health professionals, patients and patient organisations, pharmaceutical companies and policymakers to know about the legislation and processes for early access to innovative medicines. Most patients are well-informed about novel therapies due to the increase in revolutionised technologies. This enables governments to prioritise and allocate budgets, and also streamline regulations for easier access to medications.
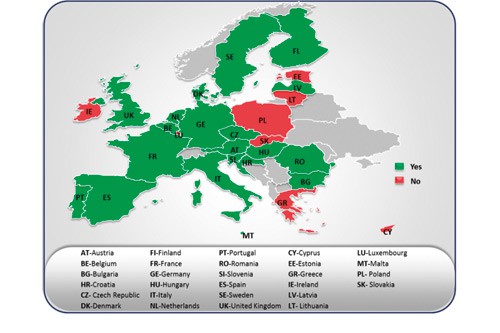
The presence of CUP in EU28 member states
The presence of CUP in EU28 is illustrated in the figure shown here. Countries like Austria, Belgium, Bulgaria, the Czech Republic, Denmark, Finland, France, Germany, Hungary, Italy, the Netherlands, Portugal, Romania, Slovenia, Spain, Sweden and the United Kingdom have nationalised regulations and well-defined processes for CUP. France has an elaborate CUP scheme called ATU (Temporary Authorisations for Use) followed by Germany, Italy, Spain and the UK.
In most countries, there are national authorities that oversee decisions and approvals for CUP. In countries that do not have CUP, the national authorities provide medicinal products either to individuals or cohorts of patients governed by each member state’s legislation. However, named patient basis is not covered under CUP as per the EU regulations, although Hungary and Sweden have recently formulated their own national legislations. Clear regulations can systematise CUP and provide better access to medicines. For instance:
- Over 20,000 patients were treated with more than 200 products under ATUs by 2007
- Studies show that the review time to obtain marketing authorisation was shortened by a combined total of 36 months
- The French ATU programme has accelerated the availability of new drugs
- Since 2006, the EU28 submitted more than 50 CUP notifications to the EMA, of which around two-fifths are for orphan drugs.
Early access programmes provide many benefits for patients and HCPs
CUP – an enabler for patient access
Investigational drugs are the last chance for patients with rare cancers when there are no other existing treatments. Early access programmes like CUP provide many benefits for patients, healthcare professionals and pharmaceutical companies. Patients unable to participate in clinical trials due to mobility issues or who fail to fulfil the eligibility criteria are able to benefit from CUP. Healthcare professionals can use CUP to provide better healthcare services for rare cancer patients, mostly with regards to symptomatic treatment and supportive or palliative care. Physicians also can use CUP to better understand rare cancers, with evidence-based practice resulting from the usage of orphan drugs at very early phases. These programmes enable pharmaceutical companies to respond more quickly and more efficiently to patient demand outside traditional access routes. CUP helps facilitate a smoother transition of orphan drugs from clinical trials to market, and also helps companies as they develop global launch strategies for global usage patterns and market landscape predictions. In addition, market authorisation holders can help to resolve any product-related issues and overcome challenges encountered by pre-approved drugs via CUP. Above all, CUP provides valuable information on real-world evidence for practice and further research.
Although CUP is beneficial for access to orphan drugs for rare cancers, it can be highly challenging and complex for governments and pharmaceutical companies to initiate. Despite the existing EU regulations, pharmaceutical companies are still challenged by the variations in legislation from country to country. Thus, it is imperative for stakeholders to be updated on the regulations and processes on CUP to facilitate easier entrance into different markets. This mandates that regulators, policymakers and other key stakeholders must streamline processes and ensure transparency.
The EU28 countries are striving to improve patient access to treatment and care of rare cancers by devising national plans and strategies. The national plans mostly leverage existing resources, encourage research, increase advocacy and improve rare cancer care through expanding networks and increased collaboration. This has led to more investments in the field of rare cancer research, which in turn has led to the development and introduction of many new orphan drugs in the recent past. These initiatives have encouraged pharmaceutical companies to develop new therapies for patients with rare, life-threatening genetic diseases. In order to continue to advance these programmes, it is vital to ensure that clearly-set regulations and rationalised procedures are in place to help patients, patient organisations and physicians to better access orphan drugs.





