 The potential of using messenger RNA as a vehicle to make any protein – within a patient’s own cells – is raising the tantalising prospect of transforming the treatment of a wide range of human diseases.
The potential of using messenger RNA as a vehicle to make any protein – within a patient’s own cells – is raising the tantalising prospect of transforming the treatment of a wide range of human diseases.
To date, most of the therapeutic applications of RNA have focused on its potential for manipulating gene expression in areas such as cancer and infectious disease, via techniques such as RNA interference (RNAi). While RNAi have shown potential in switching off specific genes and the proteins they make, attention is building the idea of using modified mRNA as a replacement therapy that can switch on production of just about any protein of interest.
The idea behind the approach is disarmingly simple, and relies on the fact that mRNA serves as an intermediary between DNA-encoding genes and their protein products. When used as a therapeutic, synthetic mRNA molecules are introduced into targets cells, whereupon the patient’s own cellular machinery (see image below) swings into action and starts producing the mRNA-encoded polypeptides and proteins.
It is envisaged that any protein type – enzymes, receptors, secreted proteins, antibodies etc – can be produced by introducing modified mRNA into the system.
Many of the scientists working on the approach describe it as gene therapy without genes, in other words achieving gene expression without actually modifying the genome, and suggest it may sidestep the safety concerns that have plagued the development of gene therapies.
Moreover, as it all happens within the cell, there is no need for mRNA drug developers to invest in large-scale bulk protein manufacturing.
“mRNA replacement opens up entirely new therapeutic areas,” according to Ali Mortazavi, chief executive of UK firm Silence Therapeutics that has just raised almost £40m ($62m) in a fundraising round.
“This technology opens up a wide range of potential treatments for genetic deficiencies, protein deficiencies, vaccines, infectious diseases and even cellular reprogramming,” he said.
mRNA technology is fundamentally disruptive, enabling us to do things not possible with current approaches
In practice of course there are still significant challenges to be overcome. The mRNA molecules need to evade the immune system unscathed – they can easily be mistaken for RNA viruses – and reach the specific cells or tissues where their protein-producing activity is desired.
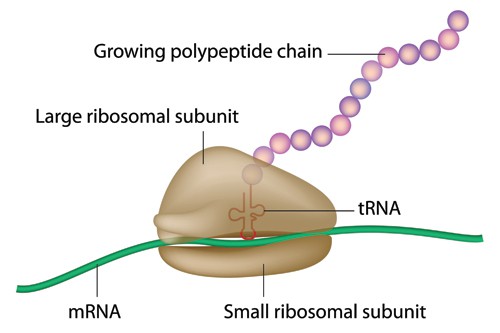
They then need to be taken up into the cells effectively and, once inside, be stable enough to produce their encoded protein for long enough to exert a therapeutic effect with a realistic dosing frequency.
In that respect mRNA is in a similar position as gene therapy and RNAi, which are only just starting to gain traction despite decades of research held back for the most part by delivery challenges.
Mortazavi said mRNA is benefiting from an increased optimism about RNA therapeutics in general, with an RNAi candidate from Alnylam due to generate phase III data later this year on its lead candidate patisiran, a treatment for rare disease familial amyloidotic polyneuropathy.
The technologies have got much better in the last few years, he said. In particular, the delivery systems are more specific and achieve better cellular uptake of the RNA payload.
mRNA molecules tend to be bigger even than RNAi candidates, he added, so there is a critical need for effective delivery technologies to get them into the right cells.
Silence and other mRNA specialists already have their own in-house technologies for delivery, while several drug delivery companies have stepped up to help other developers advance their projects. Among these are lipid nanoparticle specialists Acuity Therapeutics and Tekmira, Phase Rx with a polymer-based technology.
“Liposomal delivery systems are well suited to mRNA in comparison to other delivery technologies which are limited to smaller payloads,” according to Silence, which also says its ability to direct liposomes to different tissues – by altering their lipid content – will be a major competitive advantage.
Meanwhile, chemical modifications that have made the mRNA molecules much more stable in vitro – extending their half-life in the body to two or three days – have also made them less visible to the immune system.
For example, a research team from the University of Pennsylvania showed that substituting the nucleotide uridine in the mRNA sequence with a modified form such as 5-methylcytidine or pseudouridine dramatically reduce their immunogenicity.
| Protein replacement and activation: therapies | ||
| Company | Publicly-disclosed areas of interest | Partner(s) |
| Arcturus Therapeutics | thrombopoietin; alpha-1 antitrypsin replacment; cystic fibrosis; iron disorders | Tekmira, Arrowhead |
| BioNTech RNA Pharma | cancer, protein replacement | |
| Ethris GmbH | erythropoietin replacement; lung diseases | Shire |
| Eukarys | monogenic liver diseases; Wilson disease | |
| Moderna Therapeutics | cancer; cardiovascular disease; infectious disease; metabolism | Alexion; AstraZeneca (AZ); Karolinska Institute |
| Shire | cystic fibrosis | Cystic Fibrosis Foundation Technology |
| Silence Therapeutics | undisclosed | |
| Antigen creation: vaccines | ||
| Company | Publicly-disclosed areas of interest | Partner(s) |
| BioNTech RNA Pharma | Cancer | |
| CureVac GmbH | Cancer, infectious diseases | Boehringer Ingelheim; Johnson & Johnson (J&J); Sanofi |
| Valera LLC (Moderna Therapeutics) | Infectious diseases | Merck & Co; Pasteur Institute |
No clinical candidates… yet
mRNA therapeutics remain in the earliest stages of development but the number of candidates in preclinical testing is rising fast, as is the number of companies getting into the field and the attention they are starting to generate among investors.
One indicator of the hyperbole becoming attached to mRNA therapeutics came a few weeks ago when Moderna Therapeutics, one of the companies at the forefront of the field, was dubbed the ‘top disruptor’ among the 50 game-changing companies in CNBC’s annual survey.
That is quite an achievement considering to date neither Moderna nor its rivals in the field have advanced a drug candidate out of preclinical development or even published extensively in peer-review. However, it is one which is fully justified, according to the company’s chief executive Stéphane Bancel.
“Our mRNA technology is fundamentally disruptive, enabling us to do things not possible – or not done well enough – with current approaches,” he said, noting that the company has 50 programmes in preclinical development.
Moderna’s accolade also comes on the tail of a $500m venture financing – the largest-ever private round for a biotech – as well as a string of alliances with AstraZeneca (AZ) and Alexion in mRNA therapeutics and Merck & Co and the Pasteur Institute for mRNA-based vaccines.
Alexion said recently it has started preclinical development on seven mRNA programmes under the terms of its research pact with Moderna, with the first scheduled to enter clinical testing in 2016, while AZ’s programme is now approaching 30 separate projects.
Such is the wealth of opportunities that can be targeted by mRNA that Moderna has formed three separate business units to tap the potential. In May the company announced the creation of Elpidera to target rare diseases, joining its earlier start-ups Onkaido Theraeutics – focusing on cancer – and Valera for infectious diseases. More start-ups are in the planning stages, he added.
By the end of 2016, according to Bancel, several mRNA drugs should be in clinical testing and Moderna will start sharing its data with the scientific community.
One possible candidate is a vaccine specialist, drawing on a parallel research effort that is examining how mRNA can be used as a form of vaccination by generating antigens that can direct the host immune system to attack a specific cell or tissue type. Unsurprisingly, the approach lends itself to the treatment of diseases such as cancer, as well as infectious diseases.
Among the claimed benefits of the approach, safety and effectiveness issues sometimes associated with vaccines that are based on live attenuated viruses and recombinant viral vectors, still arise. Moreover, broadly-applicable and generic RNA manufacturing techniques are compatible with the rapid production of vaccine candidates in response to emerging infectious disease threats.
Big pharma’s involvement in mRNA – with heavyweights such as AZ, Merck, J&J, Sanofi licensing their way into the field while Novartis is still active in the field despite exiting from the RNAi sector last year – is clear evidence of the momentum building behind the technology.
“The long-held concept of a whole new class of therapeutics that can address previously undruggable targets is becoming a reality,” said Mortazavi.




