 A decision of the future location of the European Medicines Agency has moved a step closer after the closure of applications to host the agency when it leaves London.
A decision of the future location of the European Medicines Agency has moved a step closer after the closure of applications to host the agency when it leaves London.
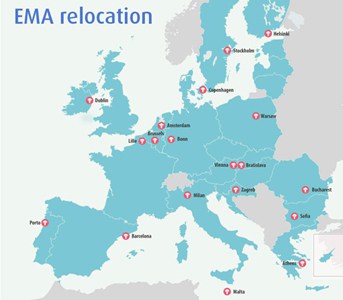
Interest in the Agency is high, with 19 of the EU’s soon-to-be 27 member states throwing their hats into the ring, with bids from Dublin, Copenhagen, Barcelona and Vienna among them.
The applications will now be assessed on the basis of six criteria, with the European Commission’s – already delayed – decision due in November.
For cities to pass the preliminary assessment stage they must guarantee the Agency will be fully operational when the UK leaves the EU and that the proposed location is easily accessible, with schools close by for the children of the staff.
Noel Wathion, EMA’s head of Brexit task force, said: “Preparing for the move, managing the necessary changes, and addressing challenges such as possible losses in skilled and experienced staff, in a proactive and efficient way requires considerable internal resources.”
Further requirements include access to the local labour market and healthcare for employees’ families, business continuity and “geographical spread”.
The same assessment criteria is in place for the European Banking Authority (EBA), which will also have to leave London after Brexit.
Belgium, France, Germany, Ireland, Czech Republic, Luxembourg, Austria and Poland have all applied to host the banking body, however, member states will only be allowed to host either the EMA or the EBA.
The European Commission will publish an assessment of the offers for the EMA and EBA by the end of September, with ministers due to then discuss the assessment in October.
Decisions of their locations will then be made in November, via a secret ballot of ministers from the 27 states that will make up the EU after March 29, 2019, with the EMA’s destination finalised first.
The full list of cities and countries offered to host the EMA
Amsterdam (The Netherlands)
Athens (Greece)
Barcelona (Spain)
Bonn (Germany)
Bratislava (Slovakia)
Brussels (Belgium)
Bucharest (Romania)
Copenhagen (Denmark)
Dublin (Ireland)
Helsinki (Finland)
Lille (France)
Milan (Italy)
Porto (Portugal)
Sofia (Bulgaria)
Stockholm (Sweden)
Malta (Malta)
Vienna (Austria)
Warsaw (Poland)
Zagreb (Croatia)




