Blake Dark, NHS England Director, Commercial Medicines introduced himself to the industry
NHS England now plays a key market access gatekeeper role for the industry’s high cost specialised medicines, and has become increasingly commercially savvy over the last few years, working alongside cost effectiveness watchdog NICE.
Late last year, NHS England went one step further to develop its pricing and reimbursement expertise and appointed Blake Dark, an experienced pharma industry executive, as the new head of its commercial medicines division.
On Wednesday, in one of his first public presentations to an industry audience, the ex-Sanofi executive set out his stall, saying that he had spent “24 years in the industry and nearly four months in the NHS, so still a little biased maybe, but let’s see”.
His appointment is part of a pattern – Steve Oldfield, another ex-Sanofi executive, was appointed by the Department of Health and Social Care (DHSC) to the new role of chief commercial officer in 2017.
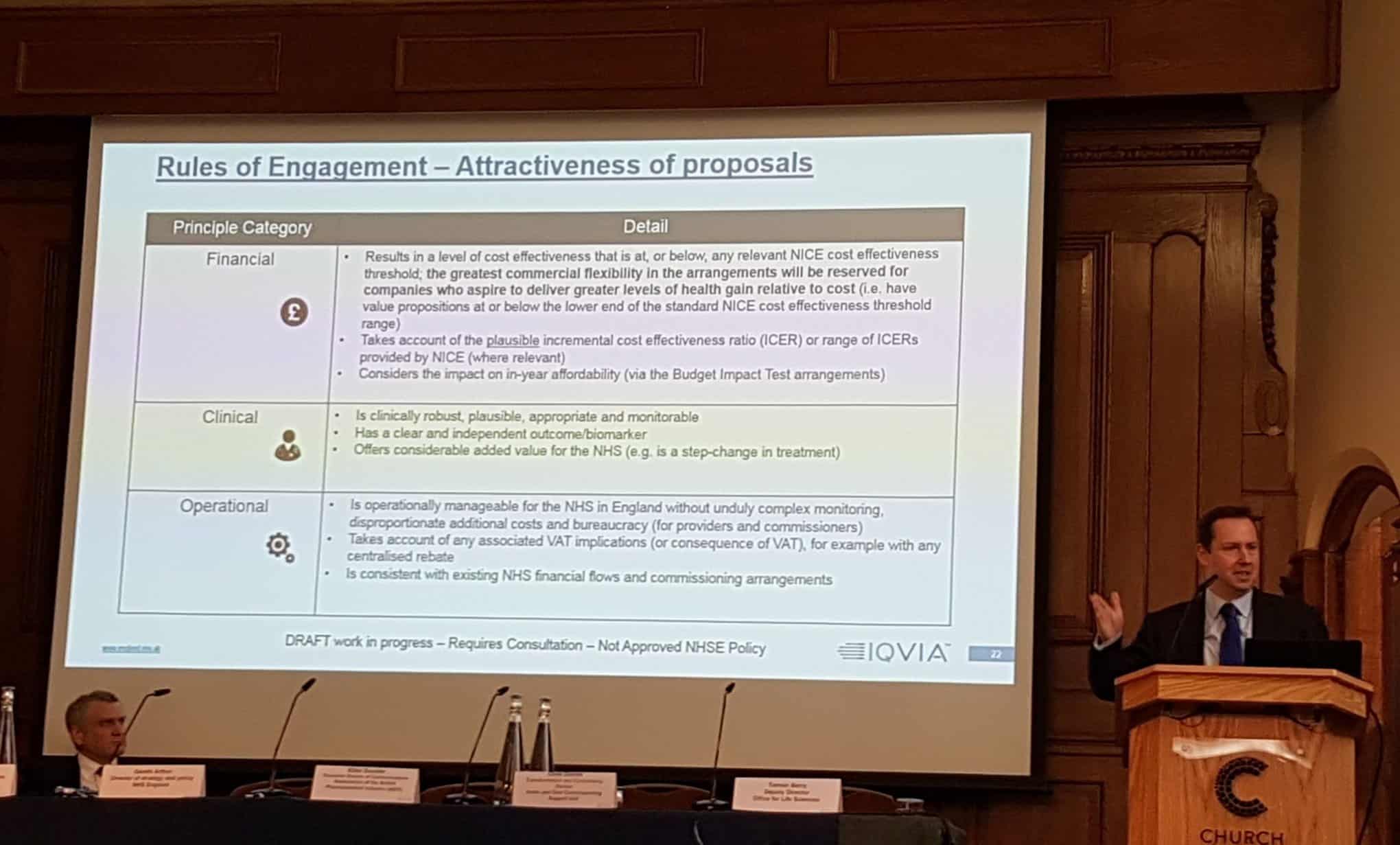
Dark’s arrival coincided with the signing of a new five-year Voluntary Price and Access Scheme (VPAS), which caps NHS expenditure on medicines, but also allows NHS England to agree individual price and access deals with pharma.
Some recent examples seen as a success on both sides are deals to bring the groundbreaking CAR-T drugs to patients – Gilead’s Yescarta and Novartis’ Kymriah gaining access in England faster than almost anywhere else in Europe.
However there have been some major disagreements as well – AbbVie took NHS England to court over its hepatitis C procurement process (losing its challenge in January), while Vertex is still deadlocked with the budgetholder over the cost of its cystic fibrosis treatment Orkambi.
Speaking at a UK market access summit hosted by IQVIA in London, Blake Dark said he was still in the early days of his post, and was listening to industry views and formulating a new commercial framework.
While this is still very much in draft form, he made it clear that it wouldn’t undermine or provide a way to bypass NICE, and neither would it compromise the principles of the VPAS deal.
Conversely, he said the use of horizon scanning would be enhanced by NHS England – including understanding the entry of biosimilars and competitor products – while also providing new opportunities for strategic partnerships and bespoke access deals.
One of Blake Dark’s key messages to the sector was that it should try to meet NICE’s cost effectiveness threshold of £30,000 per QALY at the first time of asking, rather than going above this level with the hope of negotiating a higher price.

Blake Dark: brings industry insights
In his career, spent exclusively at Sanofi, he held a number of senior posts, including spells as national sales director and commercial director in the UK and the company’s generics division, with his last role being co-ordinator of Sanofi’s diabetes and cardiovascular divisions in the Nordic and Baltic region.
Drawing on this background he said:
“I know strategically from when I was in industry, the last six months of the patent life are going to be the most valuable.
“One of my key messages is I’m not sure that spending six months in a negotiating room salami slicing your price is actually the best way to deliver value to your shareholders, I’d like you to consider coming with a price somewhere between £20,000 and 30,0000 cost per QALY, getting immediate access, and having us help deliver you innovative ways to drive your [market] penetration.”
He added: “It’s a different way of looking at it, I think, and I hope that one hits home.”
Speaking to Pharma Market Europe after his presentation, Dark said he was still very much in listening mode and getting to grips with the post, but stressed that NICE’s processes remained the first port of call for industry market access.
He also told the IQVIA conference audience that pharma companies who approach NHS England saying they have an ‘innovative’ deal should clarify what they mean by this, and whether the innovation is in the product’s clinical offering or in the reimbursement.
There is no doubt, however, that NHS England will continue to drive a hard bargain. Dark noted that costs for hospital-based drugs are forecast to increase by value at a rate of 12% CAGR over the next five years, and said NHS England would be mindful of that, maintaining a tough bargaining position, even though the VPAS scheme provides an overall ceiling to health service expenditure.




