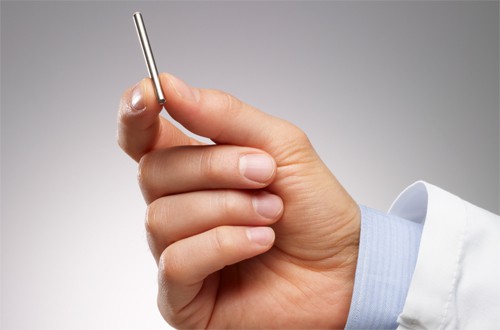
Intarcia’s ITCA 650 pump implant is the size of a matchstick
French pharma company Servier has licensed rights to a tiny drug-loaded pump device developed by Intarcia Therapeutics in a deal worth up to $1bn.
Servier has taken rights to the ITCA 650 pump – which is implanted under the skin and delivers the GLP-1 agonist exenatide – outside the US and Japan. The company said in a statement that the device “could reshape the treatment of type 2 diabetes in the very near future.”
The matchstick-sized osmotic pump would provide a continual delivery of the GLP-1 agonist – which works by improving the secretion of insulin – and would only need to be implanted once or perhaps twice a year. It is currently in phase III testing.
“A qualified physician, nurse or physician’s assistant can place ITCA 650 in a simple five-minute procedure in a doctor’s office,” said the two companies in a statement. If approved, ITCA 650 would be the first and only injection-free GLP-1 therapy to deliver up to a full year of treatment in a single dose.
Exenatide is the active ingredient in AstraZeneca’s once-daily Byetta and once-weekly Bydureon diabetes drugs, which compete in an estimated $3bn market for GLP-1 agonists against products such as Novo Nordisk’s top-selling Victoza (liraglutide) and Eli Lilly’s just-launched Trulicity (dulaglutide).
In two recently-reported phase III trials, ITCA 650 was shown to be superior to placebo in controlling blood glucose in patients with type 2 diabetes, and also significantly improved the efficacy of oral antidiabetic drugs in patients with poorly-controlled diabetes, helping 25% of them reach targets for haemoglobin A1c (HbA1c, a measure of glucose control over time).
Two additional phase III trials are ongoing and – if positive – will enable regulatory filings around the world in the first half of 2016. One of the studies is a head-to-head trial with Merck & Co’s DPP-4 inhibitor Januvia (sitagliptin), while the other is a cardiovascular outcomes study.
Under the terms of the agreement, Boston, US-based Intarcia will receive $171m upfront, with the remainder due in the form of $230m in “early” regulatory milestone payments and another $650m in the offing down the line.
Intarcia retains rights to the implant in the US and says it is in the process of seeking a licensee in Japan.
Servier is a relatively minor player in diabetes in absolute sales terms – although it does sell a range of products based on the sulfonylurea gliclazide and has a number of projects in development – so Intarcia’s decision to go with the company rather than the likes of Sanofi, AZ, Novo Nordisk, Merck & Co or Lilly might raise a few eyebrows.
However, the US biotech’s chief executive, Kurt Graves, said while it had been in negotiations with no less than 11 other pharma companies for the ITCA 650 rights, only Servier would agree to it retaining full control of the project in the US.




