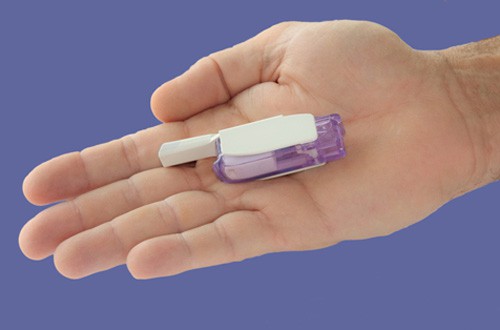
Diabetes patients in the US may have access to a new inhaled insulin in 2014 after MannKind resubmitted its regulatory filing for Afrezza to the US Food and Drug Administration (FDA).
MannKind originally submitted the drug for US approval in 2010, but faced two rejections from the FDA, with the regulator requesting further clinical trials due to concerns about the phase III data used to back the submission.
This led to MannKind’s decisions to lay off 257 employees in 2011 to better position itself to obtain the necessary data to appease the FDA.
Since then, things have improved for the company, and positive data from two phase III trials published in August this year demonstrated that the powdered insulin delivered via an inhaler is able to improve control of blood sugar in people with diabetes when added on top of oral drugs.
These studies covered the use of Afrezza in people with type 1 and type 2 diabetes and form a key part of the resubmitted New Drug Application (NDA).
“We designed the recent studies with input and guidance from the FDA, and both achieved their primary efficacy endpoints and safety objectives,” said Alfred Mann, chairman and CEO of MannKind.
The prospect of inhaled insulins provide an attractive alternative to injections for people with diabetes, but concerns about the risk of dosing errors and uptake has caused pharma companies to be wary of developing the products.
This was seen with Pfizer’s Exubera, which was withdrawn from market just one year after its launch in 2006, likely influencing Lilly and Novo Nordisk’s decisions to shelve their respective inhaled products.
MannKind is more positive though, putting its faith in the Dreamboat inhaler to be used with Afrezza, which is designed in a way to provide simpler, consistent and more accurate dosing.
“I am very proud of our team for completing an extensive submission on a very ambitious schedule,” said Mann. “We will continue to work with the FDA to bring Afrezza to market for the millions of diabetes patients in the US who might benefit from this novel product.”




