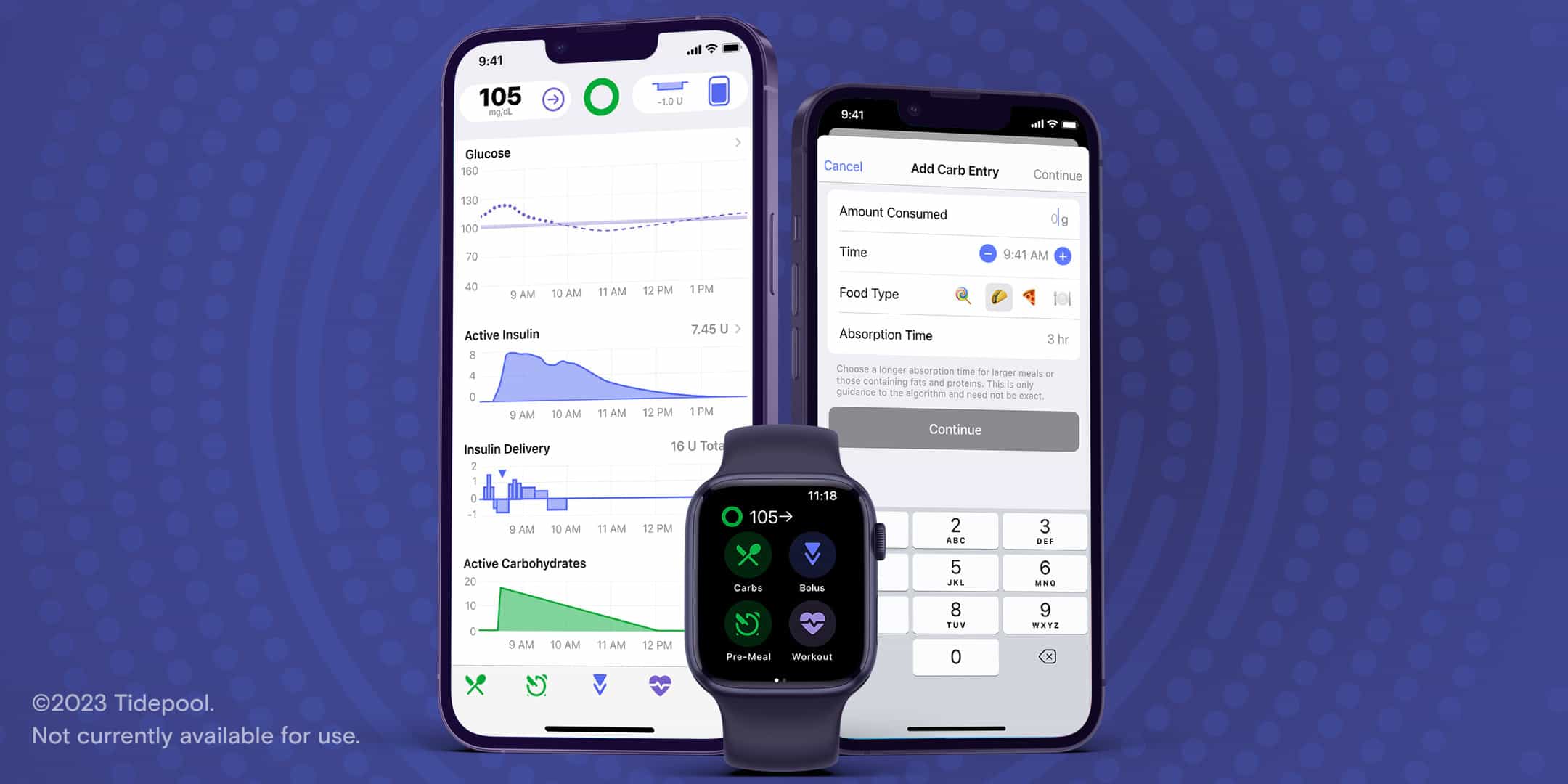
Tidepool’s automated insulin dosing app has been approved by the US Food and Drug Administration (FDA) to help those who are six years and older manage their type 1 diabetes.
Tidepool is a nonprofit organisation and Tidepool Loop is the first app of its kind to receive clearance from the FDA, as well as the first to enable insulin delivery from a compatible Apple watch.
“We knew this day would come, but we couldn’t have achieved it without the support of the entire diabetes community, including the FDA,” said Howard Look, chief executive officer and co-founder of Tidepool.
The human pancreas naturally supplies a low, continuous rate of insulin, known as basal or background insulin. In patients with diabetes however, the body’s ability to produce or respond to insulin is impaired.
Tidepool Loop’s algorithm technology is intended for use with compatible integrated continuous glucose monitors (iCGM) and alternate controller-enabled pumps to automatically increase, decrease and suspend delivery of basal insulin to the patient based on iCGM readings and predicted glucose values.
The app is also able to recommend and, with the user’s confirmation, control the delivery of correction insulin amounts when glucose values are predicted to exceed predefined thresholds.
“Tidepool helped bridge the gap between the fast pace of innovation in the community and the rigor of current quality management systems,” said David Panzirer, trustee at The Leona M and Harry B Helmsley Charitable Trust, which supported the project through a grant.
“This is a triumph for stakeholders across the diabetes industry and, most importantly, will make a real impact in the lives of people with diabetes,” Panzirer added.
The work was also supported by additional grants from JDRF, the Tullman Foundation and a community of individual funders impacted by their experience with diabetes, Tidepool said.
Aaron Kowalski, chief executive officer of JDRF, said: “Tidepool Loop’s groundbreaking FDA clearance represents a pivotal step towards a world where people with type 1 diabetes can choose the pump, CGM and algorithm that are best for them, and have all three work together.”
The Tidepool Loop app can now serve as a predicate device for future interoperable automated insulin dosing submissions, providing a more clearly defined pathway through the regulatory process.
Tidepool said it is now finalising agreements with device partners, which are yet to be announced, to create a ‘seamless experience’ for both physicians prescribing the app and the patients using it.




