Researchers in the US have started testing what they believe could be a universal vaccine against influenza, tackling both seasonal and pandemic strains such as bird flu.
The team – from the Scripps Research Institute (TSRI) in the US and Johnson & Johnson’s Janssen Pharma unit – say the vaccine could provide long-acting protection against seasonal flu, potentially doing away with the need to develop new vaccine cocktails every year to address the prevailing strains.
The ultimate aim is to have a vaccine that only has to be given every five to 10 years to protect against flu, and would also provide some protection against emerging pandemic strains.
That would include those that jump the species barrier from animals to humans, such as the swine flu epidemic that killed hundreds of thousands of people worldwide in 2009.
The work is still in animal studies but focuses on using a different part of the flu virus as the basis for the antigen used in the vaccine.
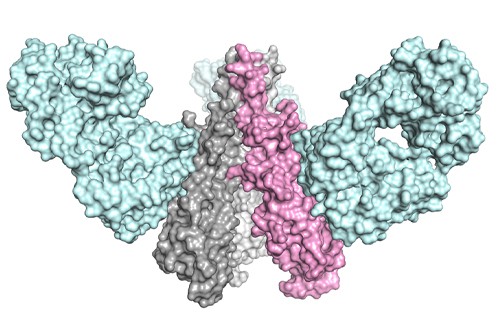
At the moment, scientists analyse the flu surface protein hemagglutinin (HA) in circulating flu strains to guess which will be causing infections during the flu season and setting in motion a manufacturing scramble to make combination vaccines that can take several months.
If the virus changes in the interim, the vaccine will be less effective. That happened last year, where the engineered vaccines only cut the chance of developing flu by around 23%. To compensate, pharma companies are developing vaccines that increase the number of strains included in the cocktail from three to four.
In a bid to sidestep that arms race, the TSRI and Janssen researchers are working with the stem of the HA protein, rather than the top end which is variable. While previous efforts to ‘behead’ the protein have been unsuccessful – leaving the stem too fragile to be used – they worked out a way to stabilise it.
The synthetic version of the stem was able to stimulate antibodies that – in animal studies – bound to HA proteins from a number of influenza viruses, including the highly infectious H5N1 strain that causes bird flu, and reduced fever in monkeys infected with a non-lethal dose of swine flu.
The next step is to complete the design of the ‘mini-HA’ component to see if it also broadly protects against other influenza strains. Then, if that work is positive, the candidate could then go into phase I trials.
“This study shows that we’re moving in the right direction for a universal flu vaccine,” said Ian Wilson, who heads the department of integrative structural and computational biology at TSRI.
Barney Graham of the National Institute of Allergy and Infectious Diseases in the US told the BBC that trials comparing the new vaccine candidate to current vaccines are expected to be lengthy, and will also require some luck.
“If there isn’t a divergent flu virus present during testing it may be difficult to show a difference,” he said.
The research is published in the journal Science.




