
By Marie Koch, Senior Consultant, Commercial Strategy & Planning, Syneos Health Consulting
Dynamic 1: the ultimate gamble
Across the biopharmaceutical industry, the likelihood of a new drug getting approved is hovering around 10%. Of those drugs that are approved, roughly 30% will see commercial success. In other words, the odds of developing an asset, getting it approved and successfully bringing it to market viability are about the same as winning a single number in roulette: 3%. Being ready for those rare, high-potential launches is the no. 1 imperative in biopharmaceutical innovation today. But maximising the potential of launch is increasingly taking a strategic backseat to managing the possibility of risk. Today, many leaders are short-changing the impact of innovation at a moment that demands both advancing best practices and making calculated recalibrations.
Four specific market dynamics are changing the future of launch:
1. A new cliff is looming: Between 2018 and 2025, branded drugs worth more than $250bn in sales will see their patents expire. This new cliff is driving SG&A pressure, corporate reorganisations and both high expectation and highly conservative launches. Marketing and sales leaders are expected to fill the gap with big returns on increasingly limited investments.
2. The pipeline is booming: Drug approvals are on a steady increase globally. Changes in approach in the US, EU and China are driving more parallel studies, more accelerated approvals and the accumulation of more real-world evidence for additional indications. The potential for overall growth is significant, but mismanaged launches could make that growth significantly uneven.
3. The science is increasingly more complex: Many of the highest-potential innovations in the pipeline answer unmet needs in largely elusive disease states. Gene and cell therapies, novel approaches to rare disease and drug replacements, like digital therapies, are about to crowd the market, creating gaps in education for physician and payer alike.
4. The economics are more complex too: Demonstrating an economic benefit is table stakes for today’s launches. Regulators, payers and large, organised customers expect to see an evidence package that includes impact on cost of illness, burden of illness, quality of life and impact on caretakers. Innovators are increasingly incorporating these health economics endpoints into phase 2 trials, and throughout phase 3 and real-world data collection.

Dynamic 2: the unprepared market
Many innovators are approaching launch with one foot on the brake and one foot on the gas. They’re holding out hope that their molecule will change the market but holding back on investment to prepare that market. This newly conservative approach means that the runway from investment to approval can be as little as six months, a tiny window made even smaller by the outsized education demands of now- common dynamics like unique biomarkers, unmet needs and priority approvals.
The changing reimbursement landscape has been a big driver of this trend with many payers automatically assigning new-to market blocks at launch to manage access to costly new products. Those blocks typically extend six months, creating time for in-depth reviews by pharmacy and therapeutics (P&T) committees. Despite these relatively new levers of cost control, early education and conditioning remain critical. Even the newest drugs will be covered based on exceptions for medical need, and preparing a market takes place on a horizon of years, not months.
By pushing the investment later and later, companies are missing out on the value creation cascade that typically starts three years before launch and increases significantly one year out.
Syneos Health recently compared the investments of 19 biopharmaceutical companies that were launching their first products. It found that companies that spent a minimum of 75% of their launch-year forecasted revenue during L-1 had higher rates of launch success. Even more importantly, not a single company that spent less than 75% of their launch-year forecasted revenue in L-1 achieved a successful launch.
As a final analysis, we compared SG&A spend of companies that had a successful launch to those companies that did not. Specifically, the median SG&A spend of successful companies was ~120% of their launch-year forecasted revenue in L-3 and L-2, ~210% in L-1 and ~330% in the launch year. In contrast, the median SG&A spend for unsuccessful companies was less than 40% of their launch-year forecasted revenue in each of the years preceding launch and only 60% in the launch year.
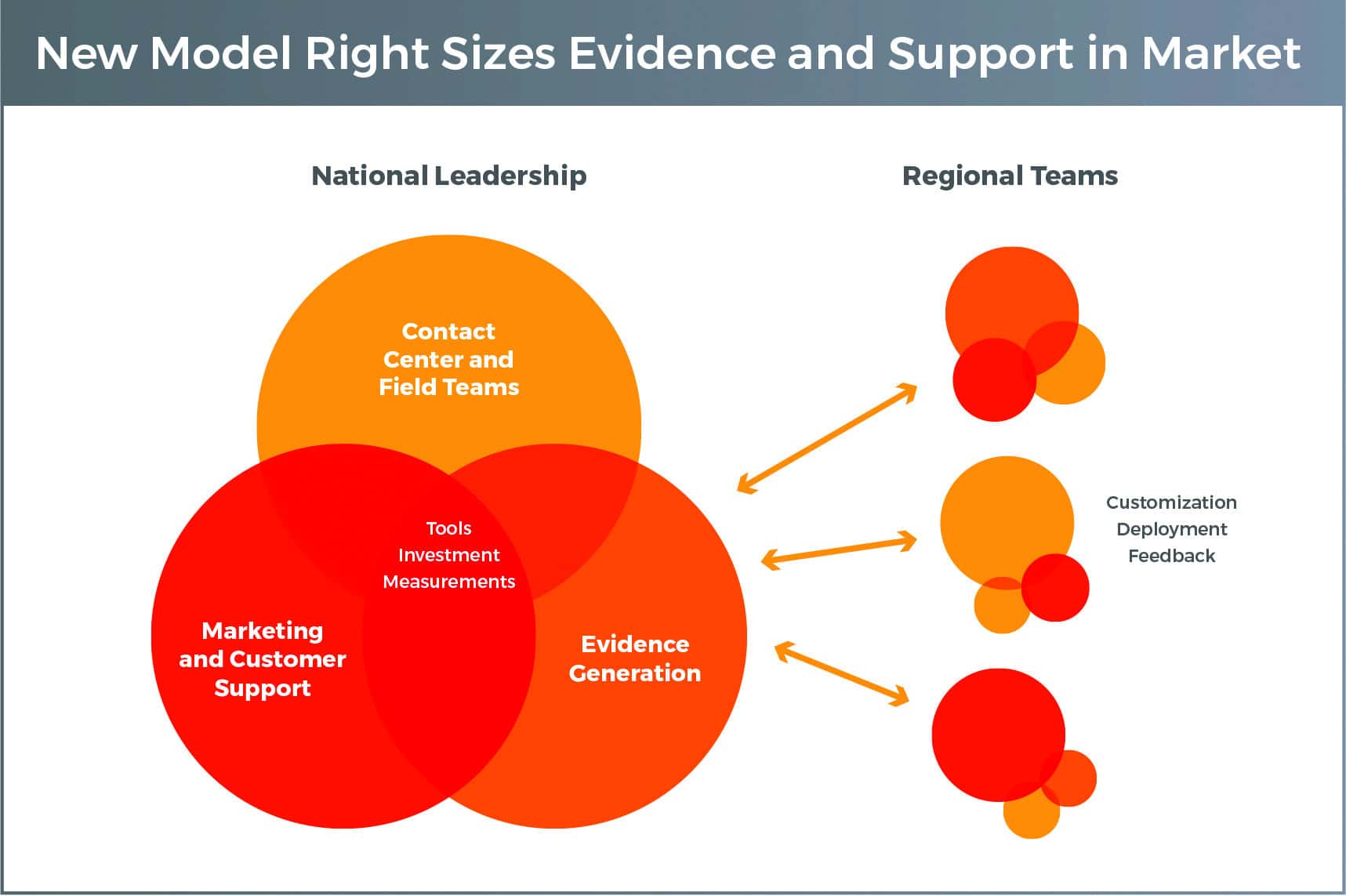
Dynamic 3: launching regionally
In 2019, the home office will start getting smaller. Pharmaceutical innovators are increasingly looking at models that push decision-making and resourcing into regional authorities at a moment when their healthcare provider counterparts are doing the same.
The NHS is restructuring to give significantly more control to seven regional directors who will be responsible for the complex triple bottom line of quality, finance and operational performance. Japan is recalibrating its traditional national quantitative approach to care decisions with a new regional approach to qualitative health and quality-of-life targets. In the US, continued consolidation of both payers and providers has created significant disparity between regions in everything from metrics to access to approach to care.
So, how do pharmaceutical companies serve these increasingly distinct regional decision-makers? They create their own responsive and right-sized regional teams. The first wave of change was giving regional sales mangers operational control of resources (not just sales accountability). In the next wave, marketing and evidence will go regional, and sales will take on an even more resource- flexible model.
Centralised global or national marketing experts will focus on building core tools, market sizing and comparative metrics. They’ll leverage market simulation and testing to develop data-driven messaging and creative packages. The regional communications lead will be a channel marketing partner to the regional sales manager, able to quickly and effectively customise and deploy content that is most relevant to regional decision-makers.
Evidence will go more regional as well. Medical affairs teams will fuel a feedback loop from providers and payers to aggregate and understand what evidence is most needed across and within regions. Some of that evidence will be advanced at a national or global level, and some will be created within region, through real-world trials that leverage nimble techniques like retrospective chart review and virtual staff.
Regional sales managers will move from making decisions on headcount to field force mix (e.g., reimbursement specialist, clinical educator, sales rep, etc.), contact centre strategy and multichannel resources. Some of those contact centres will become hybrid regional hubs where teams make outbound calls/ emails, host virtual-detail appointments and go into the field to solve problems at specific practices. Others will turn to local case managers who will be focused on navigating all regional and global resources across evidence, marketing and sales.
Dynamic 4: speed to uptake – or exit
Launches were once planned around a promised hockey stick of growth that took a low curve up the market-entry blade until it hit an inflection point and quickly started a steep climb to the blockbuster finish.
Today, increased innovation, competition and payer control have changed the expectation for sales performance. In this new era, initial value has to be realised more quickly, and sustainability needs to be projectable over time. That’s leading to new metrics that redefine performance.
For companies focused on a first or second commercial drug, those metrics also support an additional strategic component of launch: exit. In 2019, every early innovator’s launch plan needs to include strategies and associated accountabilities for proactive exit planning. Those exits can include true acquisition strategies as well as strategic outsourcing and partnerships.

This is an extract from Syneos Health’s Health Trend Ten Report, see www.syneoshealth.com/2019-health-trends





