Focus on Rare Diseases
May 10, 2017 | Medical Communications, medical education, rare diseases, recruitment
As pharma becomes more focused on rare disease therapies, Lucid Group recognises that medical education agencies need to respond.
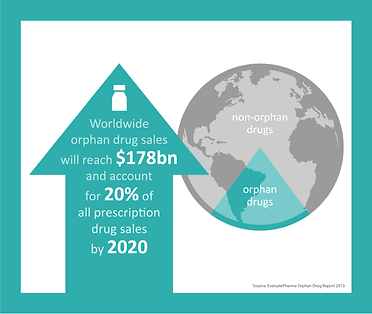
The US government passed the Orphan Drug Act in 1983, and it was subsequently adopted in other key markets, most notably in Japan in 1993 and the European Union in 2000. Growth in this sector has remained around 12% annually, which is more than double that experienced in the general drug market. According to the 2015 Orphan Drug Report, worldwide orphan drug sales will reach $178 billion by 2020 and will account for 20% of all prescription drug sales by 2020*.
The trend is clear: as science and technology advances, and the rise of the internet and social media enables patients and researchers to gather and share information more effectively, we are seeing treatments for rare diseases that only a few years ago would have led to an early death.
Alison Cantle, Rare Disease Business Unit Director at Lucid Group, explains what this means for medical education agencies: “As pharmaceutical organisations focus on personalised medicine, the need for rare disease expertise in their medical education agencies is paramount. Lucid recognised this and structured its business last year to create a unit specialising in rare diseases.”
Within this unit, Lucid Group had recruited a team of highly skilled medical writers who combine a deep and robust understanding of the science with experience of working with rare disease specialists across the globe.
“Our rare disease team understands the cornerstones of rare disease education and what makes it different from other therapy areas. It is essential to build networks and enable best practice sharing,” Alison explains. “And in rare disease, more so than in any other therapy area, the choice of agency is crucial, as often it is your agency who represents your organisation at the communication level – so its voice has to be your voice.”
Patients who have a rare disease face difficulty in every step of medical care, including diagnosis, treatment and preserving quality of life. Sometimes, patients go years without receiving the correct diagnosis for their condition. So raising awareness, improving time to diagnosis, getting patients to the right care, and improving family and emotional support and reimbursement are just some of the areas where medical education agencies can support pharma clients.
Lucid’s Rare Disease Business Unit currently works with clients in Fabry disease, Pompe disease, Gaucher disease, cystinosis and PKU. For further information about working with Lucid’s Rare Disease Business Unit, please call Alison on 01494 552078 or email alison@lucid-uk.com.
* EvaluatePharma Orphan Drug Report 2015
This content was provided by Lucid Group Communications Limited
Company Details
Latest Content from Lucid Group Communications Limited
Lucid kick-starts 2019 with spellbinding new additions to its writing and account teams
It’s been a busy start to 2019, with Lucid Group bolstering its account and medical writing teams with three new additions.Elaine Downey joins Vivid as a Senior Account Manager; she...
Lucid Group prepare for a transformative year with the appointment of a new Director of Corporate Development
Lucid Group is setting itself up for another stellar year with a new appointment to their senior leadership team. Rob Apollo has joined Lucid on a permanent basis as Director...
Lucid Group welcomes new talent into their 2019 Futures Academy
Lucid welcomed six new graduates into their training programme, The Futures Academy, this week. Launched in 2017, the Futures Academy is now a well-oiled machine, training and developing new talent...
Lucid Winter Meeting 2018
The teams gathered at The Artisan in Clerkenwell, London, to reflect on the year and celebrate both team and individual contributions to Lucid, their clients and the local community through...
2018 was a magical year for Lucid Group, filled with memorable moments and many celebrations
This year has been a ground-breaking year for Lucid Group, punctuated by magical makeovers, plenty of awards, landmark staff celebrations and worthwhile charity work.The tone of the year was set...
Lucid Group celebrate another magical year at their Winter Meeting
2018 has without doubt been a stellar year for Lucid Group, scooping a double win at Communiqué - winning both Communiqué Medical Education Consultancy of the Year and Communiqué Communications...
Lucid Group welcomes new magical talent and boosts their medical writing teams with hires in the US and UK
Lucid group welcomes four new additions to their medical writing family, with new hires across the UK and USA.Sonal Adhav joins Lighthouse Medical Communications US as a Senior Scientific Director...
Lucid attend the Wycombe BIG SLEEPOUT in aid of Wycombe Homeless Connection
The inside story from the Big Sleepout on Saturday, 24 November in support of Lucid's charity partner the Wycombe Homeless Connection.
Lucid affirms their commitment to staff development with new central services and HR hires
Lucid has recently appointed two new senior hires to their HR and central services team. Albert Capes joins as the Head of Facilities, bringing with him 11 years’ management experience...
Another spellbinding result for Lucid Group at the 2018 PMEAs
Lucid’s pioneering approach to medical education was clear to see as they scooped two top awards and a high commendation at last night’s awards ceremony.The first award was gained in...





