Focus on Rare Diseases
May 10, 2017 | Medical Communications, medical education, rare diseases, recruitment
As pharma becomes more focused on rare disease therapies, Lucid Group recognises that medical education agencies need to respond.
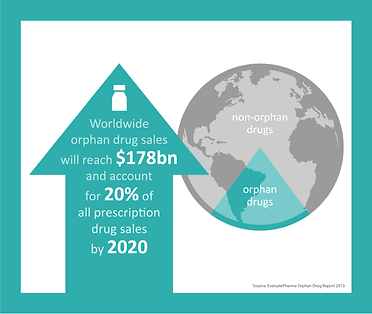
The US government passed the Orphan Drug Act in 1983, and it was subsequently adopted in other key markets, most notably in Japan in 1993 and the European Union in 2000. Growth in this sector has remained around 12% annually, which is more than double that experienced in the general drug market. According to the 2015 Orphan Drug Report, worldwide orphan drug sales will reach $178 billion by 2020 and will account for 20% of all prescription drug sales by 2020*.
The trend is clear: as science and technology advances, and the rise of the internet and social media enables patients and researchers to gather and share information more effectively, we are seeing treatments for rare diseases that only a few years ago would have led to an early death.
Alison Cantle, Rare Disease Business Unit Director at Lucid Group, explains what this means for medical education agencies: “As pharmaceutical organisations focus on personalised medicine, the need for rare disease expertise in their medical education agencies is paramount. Lucid recognised this and structured its business last year to create a unit specialising in rare diseases.”
Within this unit, Lucid Group had recruited a team of highly skilled medical writers who combine a deep and robust understanding of the science with experience of working with rare disease specialists across the globe.
“Our rare disease team understands the cornerstones of rare disease education and what makes it different from other therapy areas. It is essential to build networks and enable best practice sharing,” Alison explains. “And in rare disease, more so than in any other therapy area, the choice of agency is crucial, as often it is your agency who represents your organisation at the communication level – so its voice has to be your voice.”
Patients who have a rare disease face difficulty in every step of medical care, including diagnosis, treatment and preserving quality of life. Sometimes, patients go years without receiving the correct diagnosis for their condition. So raising awareness, improving time to diagnosis, getting patients to the right care, and improving family and emotional support and reimbursement are just some of the areas where medical education agencies can support pharma clients.
Lucid’s Rare Disease Business Unit currently works with clients in Fabry disease, Pompe disease, Gaucher disease, cystinosis and PKU. For further information about working with Lucid’s Rare Disease Business Unit, please call Alison on 01494 552078 or email alison@lucid-uk.com.
* EvaluatePharma Orphan Drug Report 2015
This content was provided by Lucid Group Communications Limited
Company Details
Latest Content from Lucid Group Communications Limited
The Futures Academy – Where magic happens and dreams, ambition and talent are realised
Findings from a recent survey by the CIPD The graduate employment gap: expectations versus reality uncovered just how hard graduates have it today. Not only are graduates earning well below...
When Dreams, Ambition and Talent Graduate
Last week saw the first intake into Lucid’s Futures Academy all graduate with full honours.
Lucid’s magic touch attracts top talent to head their HR department
Ambition and talent are in abundance with Lucid’s new hire, Angela Young, HR Director
Lucid Group spread their magic across the UK
Lucid employees can now work their magic from two new UK offices
Lucid Group bolster their commitment to agile working with the appointment of a new Head of Operations and Facilities
Thriving businesses often meet unique challenges when transitioning from a start-up to an established medium-sized company. With its numerous successes and exponential expansion over the last ten years, Lucid recognise...
Lucid’s magic touch wins them two coveted trophies at the PMEA
Lucid excel at the PMEA 2017
Magic of Lucid targets rare and specialist diseases
Lucid Group, the award-winning global medical communications company with offices in London, Buckinghamshire, Macclesfield and New York, has announced the launch of a new agency specialising in rare and specialist...
Lucid Group make the cut at the PMEAs 2017
We’re enormously proud that three of our programmes have made it to the finals at the upcoming Pharmaceutical Marketing Excellence Awards! Jan Steele COO and co-founder commented, “We are extremely...
The Lucid Group Futures Academy
A graduate programme designed to recruit the exceptional, so that we deliver the outstanding.
It’s Freshers’ week at Lucid Group
Lucid launch their Futures Academy





