Focus on Rare Diseases
May 10, 2017 | Medical Communications, medical education, rare diseases, recruitment
As pharma becomes more focused on rare disease therapies, Lucid Group recognises that medical education agencies need to respond.
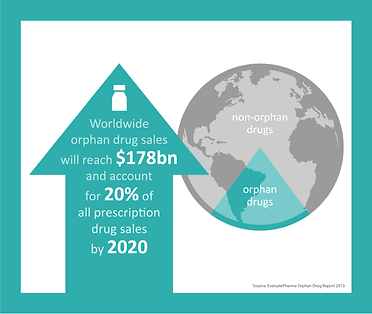
The US government passed the Orphan Drug Act in 1983, and it was subsequently adopted in other key markets, most notably in Japan in 1993 and the European Union in 2000. Growth in this sector has remained around 12% annually, which is more than double that experienced in the general drug market. According to the 2015 Orphan Drug Report, worldwide orphan drug sales will reach $178 billion by 2020 and will account for 20% of all prescription drug sales by 2020*.
The trend is clear: as science and technology advances, and the rise of the internet and social media enables patients and researchers to gather and share information more effectively, we are seeing treatments for rare diseases that only a few years ago would have led to an early death.
Alison Cantle, Rare Disease Business Unit Director at Lucid Group, explains what this means for medical education agencies: “As pharmaceutical organisations focus on personalised medicine, the need for rare disease expertise in their medical education agencies is paramount. Lucid recognised this and structured its business last year to create a unit specialising in rare diseases.”
Within this unit, Lucid Group had recruited a team of highly skilled medical writers who combine a deep and robust understanding of the science with experience of working with rare disease specialists across the globe.
“Our rare disease team understands the cornerstones of rare disease education and what makes it different from other therapy areas. It is essential to build networks and enable best practice sharing,” Alison explains. “And in rare disease, more so than in any other therapy area, the choice of agency is crucial, as often it is your agency who represents your organisation at the communication level – so its voice has to be your voice.”
Patients who have a rare disease face difficulty in every step of medical care, including diagnosis, treatment and preserving quality of life. Sometimes, patients go years without receiving the correct diagnosis for their condition. So raising awareness, improving time to diagnosis, getting patients to the right care, and improving family and emotional support and reimbursement are just some of the areas where medical education agencies can support pharma clients.
Lucid’s Rare Disease Business Unit currently works with clients in Fabry disease, Pompe disease, Gaucher disease, cystinosis and PKU. For further information about working with Lucid’s Rare Disease Business Unit, please call Alison on 01494 552078 or email alison@lucid-uk.com.
* EvaluatePharma Orphan Drug Report 2015
This content was provided by Lucid Group Communications Limited
Company Details
Latest Content from Lucid Group Communications Limited
The Lucid Winter Summit 2016
Lucid Group put their skates on this year to celebrate an outstanding year’s performance at the Lucid Winter Summit at Somerset House, London. Lucid Group enter their tenth year of...
2017: Medical education that improves value and outcomes for patients
In December’s Issue of PME, two of Lucid’s senior management team – Shula Sarner and Tanya Goodyear – consider how medical education will evolve in 2017. They discuss industry...
Lucid Group kick off their Winter Festival
The start of December not only signalled the official calendar countdown to Christmas, but also marked the official launch of the Lucid Winter Festival, which culminates in the Winter Summit...
Leading Edge expands their senior medical writing team
Leading Edge, part of the Lucid Group specialising in strategic health communications and medical education, has bolstered its senior medical writing team with the new appointment of Jennifer (Jennie) Badger. Jennie...
Lucid win PMEA Support Agency of the Year 2016
Last night the Lancaster London Hotel hosted the annual PMEA awards. These awards recognise and commend companies and programmes demonstrating excellence, best practice and innovation in improving patient outcomes on...
Lucid strengthens its creative services department
There’s no slowing down for Lucid Group as it completes its first decade of trading early next year. In parallel with the recent appointments in its senior leadership team, Lucid...
Changing behaviour: putting theory into practice
In the November issue of PME, Madeleine Tye (senior account manager at Lucid Group) discusses Lucid’s approach to medical education. Lucid employs the outcomes of research into behavioural science in...
Tajinder’s #LucidLife
Medical writers are like gold dust, so when we find talented writers with great experience,we offer a tailor-made package.Are you a world-class medical writer?Do you like the look of Tajinder’s...
Fiona’s #LucidLife
Meet Fiona, a medical writer.She told us what she wanted, and we did the rest.We believe that medical writers are like gold dust; so when we find talent, we do...
Lucid Group: What medical communications means to us
Katherine Duxbury, Scientific Director at Lucid, explains what medical communications means to Lucid: What we do, how we do it and what makes us different.





