Focus on Rare Diseases
May 10, 2017 | Medical Communications, medical education, rare diseases, recruitment
As pharma becomes more focused on rare disease therapies, Lucid Group recognises that medical education agencies need to respond.
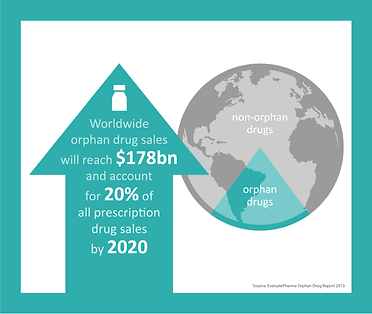
The US government passed the Orphan Drug Act in 1983, and it was subsequently adopted in other key markets, most notably in Japan in 1993 and the European Union in 2000. Growth in this sector has remained around 12% annually, which is more than double that experienced in the general drug market. According to the 2015 Orphan Drug Report, worldwide orphan drug sales will reach $178 billion by 2020 and will account for 20% of all prescription drug sales by 2020*.
The trend is clear: as science and technology advances, and the rise of the internet and social media enables patients and researchers to gather and share information more effectively, we are seeing treatments for rare diseases that only a few years ago would have led to an early death.
Alison Cantle, Rare Disease Business Unit Director at Lucid Group, explains what this means for medical education agencies: “As pharmaceutical organisations focus on personalised medicine, the need for rare disease expertise in their medical education agencies is paramount. Lucid recognised this and structured its business last year to create a unit specialising in rare diseases.”
Within this unit, Lucid Group had recruited a team of highly skilled medical writers who combine a deep and robust understanding of the science with experience of working with rare disease specialists across the globe.
“Our rare disease team understands the cornerstones of rare disease education and what makes it different from other therapy areas. It is essential to build networks and enable best practice sharing,” Alison explains. “And in rare disease, more so than in any other therapy area, the choice of agency is crucial, as often it is your agency who represents your organisation at the communication level – so its voice has to be your voice.”
Patients who have a rare disease face difficulty in every step of medical care, including diagnosis, treatment and preserving quality of life. Sometimes, patients go years without receiving the correct diagnosis for their condition. So raising awareness, improving time to diagnosis, getting patients to the right care, and improving family and emotional support and reimbursement are just some of the areas where medical education agencies can support pharma clients.
Lucid’s Rare Disease Business Unit currently works with clients in Fabry disease, Pompe disease, Gaucher disease, cystinosis and PKU. For further information about working with Lucid’s Rare Disease Business Unit, please call Alison on 01494 552078 or email alison@lucid-uk.com.
* EvaluatePharma Orphan Drug Report 2015
This content was provided by Lucid Group Communications Limited
Company Details
Latest Content from Lucid Group Communications Limited
Lucid celebrate big wins at PMEA 2015
Success for Lucid at PMEA 2015
Lucid invests in their Futures programme with a new hire
Lucid Group continues their expansion
Janus kinase inhibitors are amongst the most read abstracts at the American College of Rheumatology 2015
Rheumatologists eagerly await trial data for Janus kinase inhibitors
Making a difference to patients with IBD
Changing lives with medical education
Lucid’s motivation to make a difference in medical education drives everything they do
Changing lives with medical education
Lucid Group meets with next generation MedEd talent at University of Oxford Internships Fair
Lucid Group will welcome interns in summer 2016
Lucid Group appoints new MD for Leading Edge
New MD at Leading Edge
Lucid Group showcases new KOL identification and mapping tool
Advancing Expert identification with digital new tool from Lucid Group
Lucid CEO discusses his inspiration and passion for changing patients’ lives
Changing patients' lives with innovative methodologies in medical education
Lucid Group starts new trend for CME in IBD education
A tailored educational event in IBD





