Focus on Rare Diseases
May 10, 2017 | Medical Communications, medical education, rare diseases, recruitment
As pharma becomes more focused on rare disease therapies, Lucid Group recognises that medical education agencies need to respond.
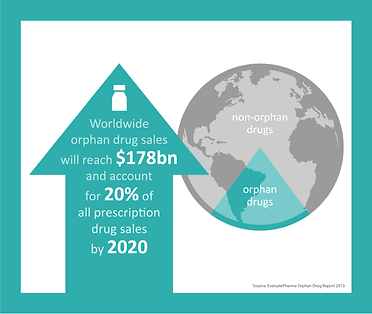
The US government passed the Orphan Drug Act in 1983, and it was subsequently adopted in other key markets, most notably in Japan in 1993 and the European Union in 2000. Growth in this sector has remained around 12% annually, which is more than double that experienced in the general drug market. According to the 2015 Orphan Drug Report, worldwide orphan drug sales will reach $178 billion by 2020 and will account for 20% of all prescription drug sales by 2020*.
The trend is clear: as science and technology advances, and the rise of the internet and social media enables patients and researchers to gather and share information more effectively, we are seeing treatments for rare diseases that only a few years ago would have led to an early death.
Alison Cantle, Rare Disease Business Unit Director at Lucid Group, explains what this means for medical education agencies: “As pharmaceutical organisations focus on personalised medicine, the need for rare disease expertise in their medical education agencies is paramount. Lucid recognised this and structured its business last year to create a unit specialising in rare diseases.”
Within this unit, Lucid Group had recruited a team of highly skilled medical writers who combine a deep and robust understanding of the science with experience of working with rare disease specialists across the globe.
“Our rare disease team understands the cornerstones of rare disease education and what makes it different from other therapy areas. It is essential to build networks and enable best practice sharing,” Alison explains. “And in rare disease, more so than in any other therapy area, the choice of agency is crucial, as often it is your agency who represents your organisation at the communication level – so its voice has to be your voice.”
Patients who have a rare disease face difficulty in every step of medical care, including diagnosis, treatment and preserving quality of life. Sometimes, patients go years without receiving the correct diagnosis for their condition. So raising awareness, improving time to diagnosis, getting patients to the right care, and improving family and emotional support and reimbursement are just some of the areas where medical education agencies can support pharma clients.
Lucid’s Rare Disease Business Unit currently works with clients in Fabry disease, Pompe disease, Gaucher disease, cystinosis and PKU. For further information about working with Lucid’s Rare Disease Business Unit, please call Alison on 01494 552078 or email alison@lucid-uk.com.
* EvaluatePharma Orphan Drug Report 2015
This content was provided by Lucid Group Communications Limited
Company Details
Latest Content from Lucid Group Communications Limited
The Lucid Group Guide
We work hard at Lucid Group. Transforming lives, it's not a walk in the park. We’re a team. In fact, we are many teams. Working together to discover, define, design,...
Our #LucidLife
We asked our people: Why do you do what you do? - What makes you get out of bed in the morning? The responses had a common theme - it's...
Lucid Group opens our new space in Marlow
This week marks an important step for everyone here at Lucid Group, as we re-open our premises in the UK and US and evolve to a hybrid way of working....
A personal perspective on autism
The whole month of April is Autism Awareness Month. To help mark the end of such an important month we’re sharing a personal perspective to spread kindness and autism awareness:When...
Lucid Group is building its data analytics capability
Lucid Group is building a new data analytics capability into its teams with the appointment of Darren Selmes as its new analyst. This appointment signals the start of a stronger...
Lucid Group promotes Clare Reynolds to Director of Business Development and Commercial Integration
As Lucid continues to evolve, its people have remained at the heart of its success. Clare Reynolds has played a key part in Lucid’s journey for nearly 12 years, during...
A magic milestone: Sally’s 10-year #LucidLife workiversary
Our people define our culture and there is no better example of what we are striving to achieve than what Sally Ratcliffe, Scientific Director, Lucid Group, has demonstrated over the...
Virtually, the same: Is it time to rethink congress strategy?
In this month’s PME, Jonathan Andrew, Technology Director at Lucid Group discusses the digital transformation that swept through the medcomms industry due to the COVID-19 pandemic. In particular, we saw...
Lucid Group Bolster its Senior Leadership Team
Lucid Group are delighted to welcome Jo Troman to the role of Managing Partner for team Origins. Jo has a wealth of industry experience from both client and agency sides....
Lucid Group Support Autism Awareness Day
Annually, 2 April is Autism Awareness Day. Being autistic does not mean you have an illness or disease; it means your brain works in a different way from other people....





