Focus on Rare Diseases
May 10, 2017 | Medical Communications, medical education, rare diseases, recruitment
As pharma becomes more focused on rare disease therapies, Lucid Group recognises that medical education agencies need to respond.
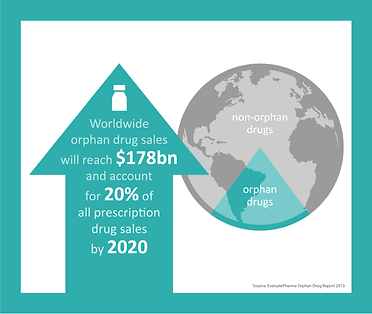
The US government passed the Orphan Drug Act in 1983, and it was subsequently adopted in other key markets, most notably in Japan in 1993 and the European Union in 2000. Growth in this sector has remained around 12% annually, which is more than double that experienced in the general drug market. According to the 2015 Orphan Drug Report, worldwide orphan drug sales will reach $178 billion by 2020 and will account for 20% of all prescription drug sales by 2020*.
The trend is clear: as science and technology advances, and the rise of the internet and social media enables patients and researchers to gather and share information more effectively, we are seeing treatments for rare diseases that only a few years ago would have led to an early death.
Alison Cantle, Rare Disease Business Unit Director at Lucid Group, explains what this means for medical education agencies: “As pharmaceutical organisations focus on personalised medicine, the need for rare disease expertise in their medical education agencies is paramount. Lucid recognised this and structured its business last year to create a unit specialising in rare diseases.”
Within this unit, Lucid Group had recruited a team of highly skilled medical writers who combine a deep and robust understanding of the science with experience of working with rare disease specialists across the globe.
“Our rare disease team understands the cornerstones of rare disease education and what makes it different from other therapy areas. It is essential to build networks and enable best practice sharing,” Alison explains. “And in rare disease, more so than in any other therapy area, the choice of agency is crucial, as often it is your agency who represents your organisation at the communication level – so its voice has to be your voice.”
Patients who have a rare disease face difficulty in every step of medical care, including diagnosis, treatment and preserving quality of life. Sometimes, patients go years without receiving the correct diagnosis for their condition. So raising awareness, improving time to diagnosis, getting patients to the right care, and improving family and emotional support and reimbursement are just some of the areas where medical education agencies can support pharma clients.
Lucid’s Rare Disease Business Unit currently works with clients in Fabry disease, Pompe disease, Gaucher disease, cystinosis and PKU. For further information about working with Lucid’s Rare Disease Business Unit, please call Alison on 01494 552078 or email alison@lucid-uk.com.
* EvaluatePharma Orphan Drug Report 2015
This content was provided by Lucid Group Communications Limited
Company Details
Latest Content from Lucid Group Communications Limited
Lucid Group sets the vision for its team of committed Mental Health First Aiders
As we approach World Mental Health Day, Lucid's team of Mental Health First Aiders reflect on their training and set the vision for how they will be supporting the organisation...
The Lucid Group Wellbeing Champions embark on their MHFA training
A mentally sound organisation is good for talent and business. Statistics from Mental Health First Aid (MHFA) England point out to mental health illness (such as depression or anxiety) accounting...
Lucid Group Nurtures Talent. Always.
Lockdown and Covid-19 have not stopped us from nurturing and developing the amazing talent we have at Lucid. Growth is at the heart of #LucidLife.So if you're looking for a place...
Lucid Group reaches the finals of the PM360 2020 Trailblazer Initiative Awards
Lucid Group has been named as a finalist in the PM360 2020 Trailblazer Initiative Awards in the HCP Education category, for the program LiBRA 2019: Looking beyond the joint in...
It’s been ten magical years at Lucid Group for Emma Cox
August marks a special work anniversary for Emma Cox who is celebrating 10 years at Lucid Group!Over the past decade, Emma has demonstrated resilience, tenacity and grit with a disarming...
Lucid Group: Digital Delivery in Lockdown
Standing still is the fastest way of moving backwards in today's world. That's why we've been moving forward and transforming at every step to make sure we can continue to...
Lucid Group scoop a win and a high commendation at Communiqué 2020
Due to the COVID-19 pandemic, this year’s Communiqué awards was a virtual ceremony, celebrating some of the most inspiring work and people in medical communications.Lucid Group were delighted to come...
PHARMA POST COVID-19: REVOLUTION OR EVOLUTION?
Post-COVID-19 thinking: outputs from a thought leader forumOn 14 May 2020, a group of industry experts across pharma and biotech Zoomed together to share reflections on the impact of COVID-19...
Lucid Group are shortlisted in three categories for Communiqué 2020
Lucid’s talent for creating industry-leading programmes is clear to see as they celebrate three of its campaigns being shortlisted for Communiqué 2020, as well as making the finals forThe Real...
Taking the ‘free’ out of ‘freelance’ and bringing it in-house
In this month’s Smart Thinking blog on PMLive.com, Stephanie Wasek, now a permanent principal medical writer at Lucid Group, draws her experience to discuss the highs and lows of being...





