
Bluebird Bio’s gene therapy treatment for beta thalassemia Zynteglo is to be priced at €1.575m ($1.76m) in Europe, where the first patients are expected to be treated later this year.
This total treatment cost will be split up into five instalments of €315,000 over five years, with payments 2-5 only made if patients continue to achieve independence from transfusion.
This plan has much in common with the outcomes-based instalment plan put forward by Novartis for its SMA gene therapy Zolgensma, which gained approval last month in the US.
Zolgensma is priced even higher, at $2.12m, but both companies are winning over payers with their similar instalment payment plans, and argue that these costs work out lower than equivalent rare disease treatments which have to be taken lifelong.
The US biotech revealed the price of Zynteglo (autologous CD34+ cells encoding βA-T87Q-globin gene) to Pharma Market Europe in an briefing with its head of Europe, Andrew Obenshain, ahead of the firm’s analyst call from the EHA congress in Amsterdam.
The one-time gene therapy was approved in Europe earlier this month for patients 12 years and older with transfusion-dependent β-thalassaemia (TDT) and have no matching donor for a stem cell transplant.
The therapy has been shown in a series of small studies to free a majority of patients from the need to have these regular blood transfusions, results which could represent a cure in the long term.
As the therapy is only currently licensed for use in beta thalassemia patients who have no matching stem cell donor, Obershain says this would make benchmarking its price against the cost of a stem cell transplant ($500,000- $1m) inappropriate.
He says the company is currently filing its pricing and reimbursement dossiers in all the major European markets, and expects to launch first in Germany, followed by the UK, France and Italy. He says the pricing plan has been well received by Europe’s payers so far.

Andrew Obenshain
“The reception that we’re getting from the payers, and the governments on this type of model is very positive. They haven’t worked out all the details yet, in terms of how it works administratively. But whereas the conversation was very one-sided a year ago with us proposing, now it is very much a two sided dialogue.”
Zynteglo won’t be the first ever €1m plus gene therapy launched in Europe – that distinction went to UniQure’s rare disease treatment Glybera, which was launched in 2012. However underwhelming clinical performance, severe restrictions around manufacturing and administration, and a single hefty upfront payment meant the drug was a commercial failure, and was withdrawn in 2017.
Bluebird has learnt from that failure, and has been careful to lay the groundwork for its outcomes-based payment model with healthcare payers. It has named its mission as ‘recoding the DNA of healthcare’ with its model likely to be adopted by subsequent gene therapy products.
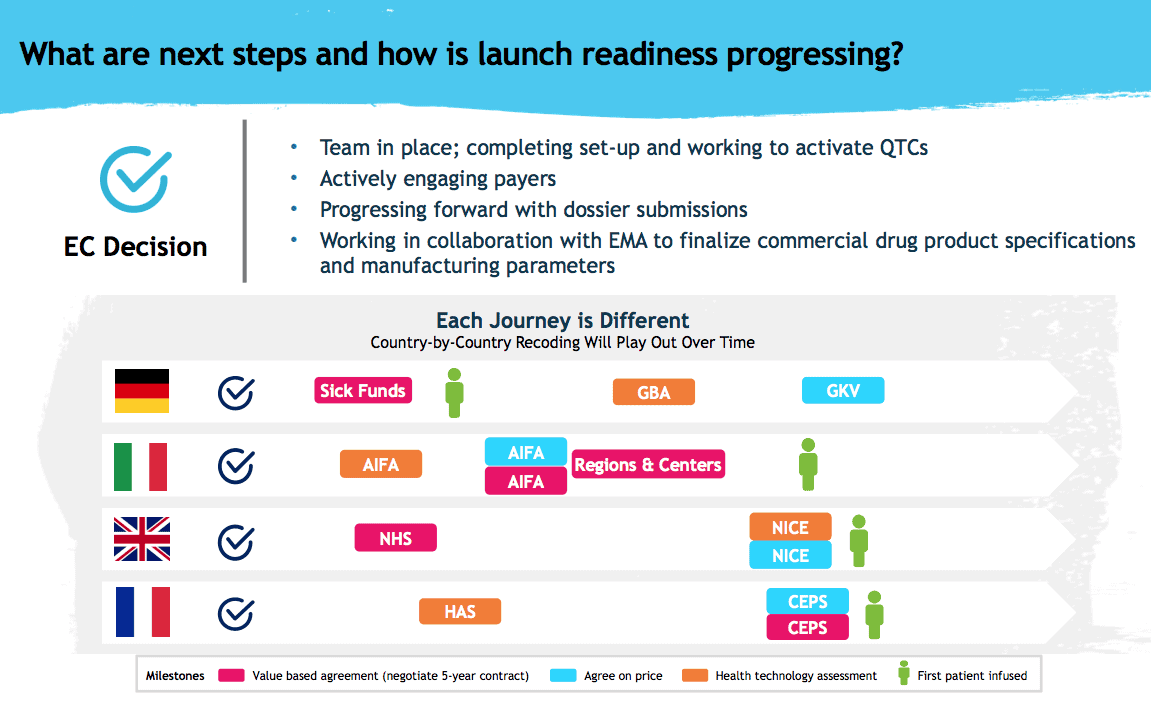
A slide from today’s presentation from Bluebird on the Zynteglo launch in Europe
“We’re trying to create a new business model for gene therapy,” says Obenshain. “It’s something we have got to do – the last thing we wanted was to cave to the existing system. A one time [payment model], that’s not sustainable for the system. Our goal is to make this sustainable for our current products, for our future products and for gene therapy in general, including those of other companies as well.”
Analysts William Blair estimated last year that Zynteglo global revenues could exceed $800m in TDT, but this will depend on whether data shows it can provide curative treatment in the harder-to- treat sub-groups.




